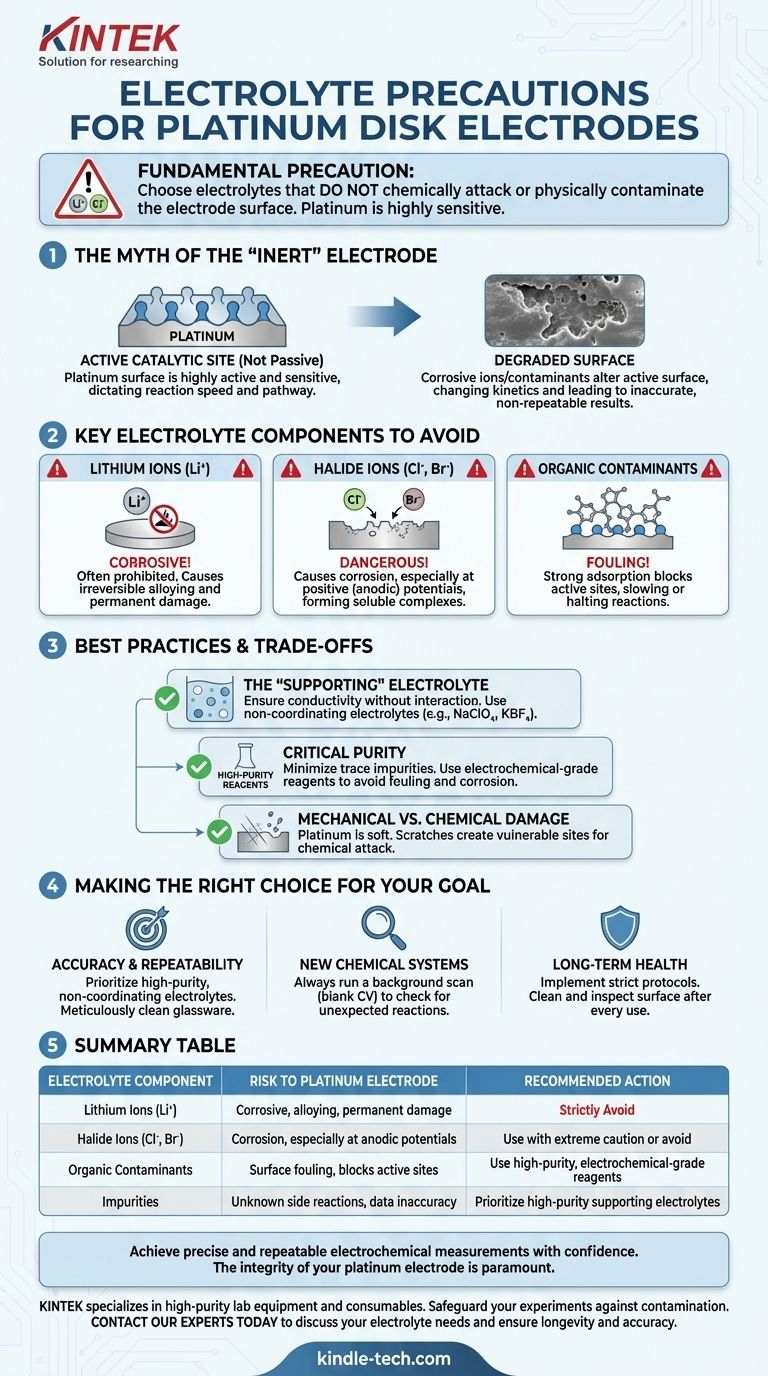
The fundamental precaution when using a platinum disk electrode is to select an electrolyte that will not chemically attack or physically contaminate the electrode surface. Platinum is less inert than often assumed, and certain ions can cause irreversible corrosion or fouling, which compromises the integrity of your data and the longevity of the electrode. Specifically, you must avoid any contact with lithium ions and be highly cautious with halide ions like chloride.
Your platinum electrode's performance is entirely dependent on the condition of its surface. The primary goal is to choose an electrolyte that provides conductivity without interfering, ensuring that the electrochemical behavior you measure is from your experiment, not from unintended side reactions or electrode degradation.

The Myth of the "Inert" Electrode
Many think of platinum as a completely inert material, but in electrochemistry, this is a dangerous oversimplification. The surface is highly active and sensitive to its chemical environment.
Platinum's Catalytic Nature
A platinum electrode surface is not just a passive conductor of electrons. It is a highly active catalytic site where electrochemical reactions take place. The precise state of this surface dictates the speed and pathway of these reactions.
How Electrolytes Degrade Performance
When an electrolyte contains corrosive ions or adsorbing contaminants, it directly alters the electrode's active surface. This doesn't just cause physical damage; it fundamentally changes the kinetics of the reaction you are trying to study, leading to inaccurate and non-repeatable results.
Key Electrolyte Components to Avoid
To maintain the integrity of the platinum surface, certain chemical species must be strictly controlled or eliminated from your electrolyte solution.
The Prohibition on Lithium Ions
The references are explicit: lithium ions (Li⁺) are corrosive to platinum. Their use is often prohibited by electrode manufacturers. Alloying between lithium and platinum can occur, permanently damaging the electrode structure.
The Danger of Halide Ions (Cl⁻, Br⁻)
Halide ions, particularly chloride, are notorious for causing corrosion of platinum, especially at positive (anodic) potentials. They can form stable, soluble platinum-halide complexes, effectively stripping platinum atoms from the electrode surface over time.
Contamination from Organic Substances
Many organic molecules can strongly adsorb onto the platinum surface. This process, known as fouling, blocks the active sites needed for your desired reaction, which can dramatically slow down or halt the electrochemical process you are measuring.
Understanding the Trade-offs and Best Practices
Choosing the right electrolyte involves more than just avoiding harmful substances. It requires a proactive approach to maintaining a pristine electrochemical environment.
The Role of a "Supporting" Electrolyte
The primary function of the electrolyte is to ensure the solution is conductive. An ideal supporting electrolyte contains ions that are electrochemically inactive in your potential window and do not interact with the electrode surface. This is why non-coordinating electrolytes based on salts like perchlorates (e.g., NaClO₄) or tetrafluoroborates (e.g., KBF₄) are often preferred.
The Critical Importance of Purity
Trace impurities in your solvent or electrolyte salt can be a major source of contamination. Always use high-purity, electrochemical-grade reagents whenever possible to minimize the risk of introducing unknown species that could foul or corrode your electrode.
Mechanical vs. Chemical Damage
Remember that platinum is a soft metal. Mechanical damage from scratches or impacts creates high-energy surface sites. These damaged areas are often more susceptible to chemical attack and corrosion from the electrolyte than a smooth, polished surface.
Making the Right Choice for Your Goal
Your experimental objective should guide your level of precaution and your choice of materials.
- If your primary focus is accuracy and repeatability: Prioritize high-purity, non-coordinating electrolytes and meticulously clean your glassware to avoid any sources of halide or organic contamination.
- If you are investigating new chemical systems: Always run a background scan (e.g., a blank cyclic voltammogram) with just your electrode and the new electrolyte to check for unexpected reactions before adding your analyte.
- If your goal is long-term electrode health: Create a checklist of prohibited substances for your lab, and implement a strict protocol for cleaning and inspecting the electrode surface after every use.
Ultimately, a well-chosen electrolyte is the foundation for producing reliable and trustworthy electrochemical data.
Summary Table:
| Electrolyte Component | Risk to Platinum Electrode | Recommended Action |
|---|---|---|
| Lithium Ions (Li⁺) | Corrosive, can cause alloying and permanent damage | Strictly avoid |
| Halide Ions (Cl⁻, Br⁻) | Corrosion, especially at anodic potentials | Use with extreme caution or avoid |
| Organic Contaminants | Surface fouling, blocks active sites | Use high-purity, electrochemical-grade reagents |
| Impurities | Unknown side reactions, data inaccuracy | Prioritize high-purity supporting electrolytes (e.g., perchlorates) |
Achieve precise and repeatable electrochemical measurements with confidence. The integrity of your platinum electrode is paramount to your research. KINTEK specializes in providing high-purity lab equipment and consumables, including electrochemical-grade reagents and reliable electrodes, to safeguard your experiments against contamination and corrosion. Contact our experts today to discuss your specific electrolyte needs and ensure the longevity and accuracy of your electrochemical setup.
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Electrolytic Electrochemical Cell for Coating Evaluation
- Flat Corrosion Electrolytic Electrochemical Cell
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
People Also Ask
- What is the proper post-treatment procedure for a platinum sheet electrode? Ensure Long-Term Accuracy & Protect Your Investment
- How should a platinum sheet electrode be operated during an experiment? Ensure Accurate and Reproducible Results
- What are the performance characteristics of platinum sheet electrodes? Unlock Superior Electrochemical Performance
- How should a platinum sheet electrode be pretreated before use? Ensure Accurate Electrochemical Measurements
- What are the key performance characteristics and applications of platinum sheets? Unmatched Reliability for Demanding Applications



















