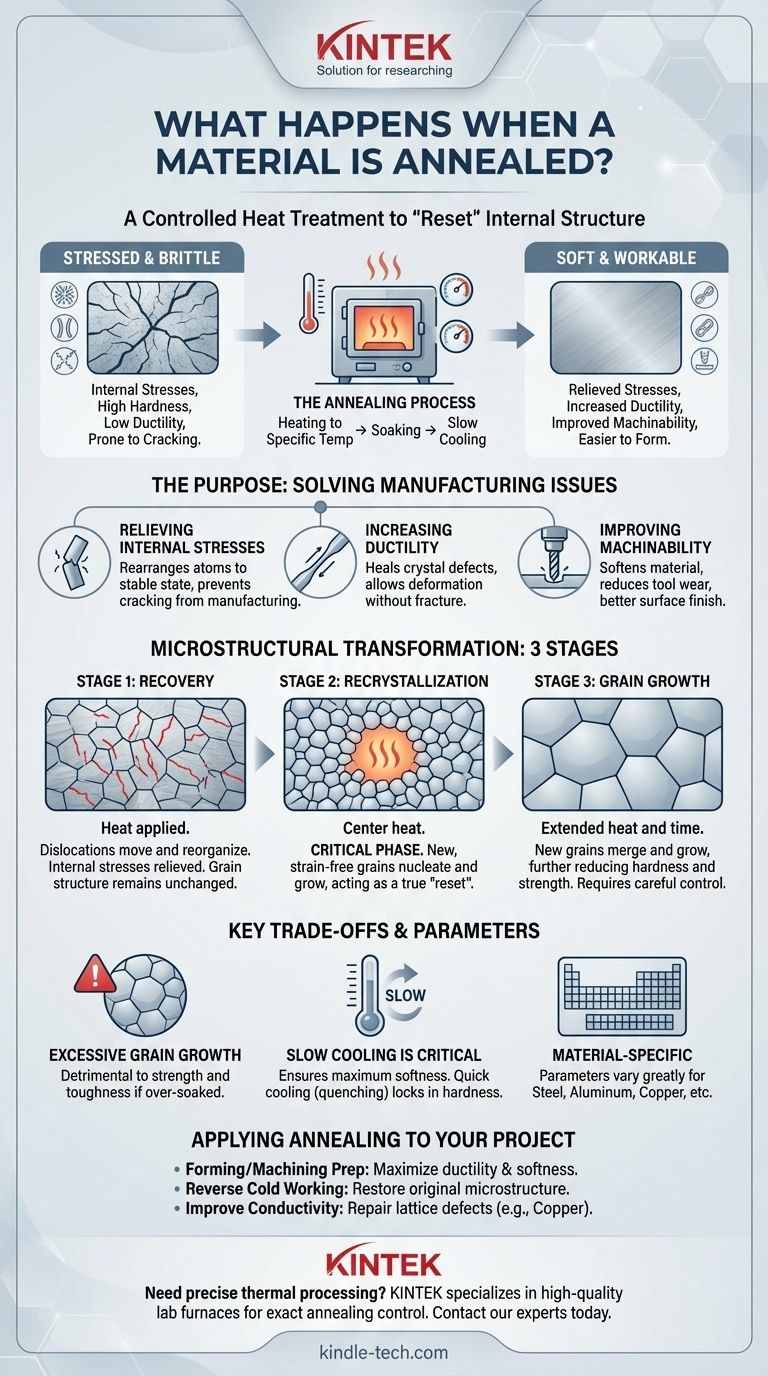
In essence, annealing is a controlled heat treatment process designed to "reset" a material's internal structure. It involves heating a material like steel, copper, or aluminum to a specific temperature, holding it there for a time, and then cooling it slowly. This procedure alters the material's physical and sometimes chemical properties, making it softer, more ductile, and easier to work with.
The fundamental goal of annealing is to relieve internal stresses and remove microscopic defects within a material's crystal structure. This process trades hardness for increased ductility and machinability, transforming a hard, brittle material into one that is soft and workable.

The Purpose: From Stressed and Brittle to Soft and Workable
Annealing is not performed arbitrarily; it is a solution to specific problems introduced during manufacturing processes like casting, forging, or cold working (e.g., bending or drawing).
Relieving Internal Stresses
Processes that deform a material at room temperature create significant internal stresses within its crystalline structure. These stresses can make the material prone to cracking or unpredictable failure over time. Annealing provides the thermal energy needed for the atoms to rearrange into a more stable, lower-stress state.
Increasing Ductility
Ductility is a material's ability to be stretched or deformed without breaking. By "healing" the defects in the crystal structure, annealing makes a material significantly more ductile. This is critical before processes like stamping, drawing wire, or deep forming, where a brittle material would simply fracture.
Reducing Hardness and Improving Machinability
There is an inverse relationship between hardness and ductility. The structural reset from annealing makes the material softer. This reduction in hardness directly improves machinability, meaning it is easier to cut, drill, or shape, resulting in less tool wear and better surface finishes.
The Three Stages of Microstructural Change
At a microscopic level, annealing is a precise, three-act transformation. These stages are what fundamentally change the material's properties.
Stage 1: Recovery
As the material is heated, it first enters the recovery stage. At this lower temperature, the material begins to soften as thermal energy allows linear defects, known as dislocations, to move and organize into lower-energy arrangements. This process relieves much of the internal stress, but the overall grain structure of the material remains unchanged.
Stage 2: Recrystallization
This is the most critical phase. As the material is held at its target annealing temperature (a process called "soaking"), new, strain-free grains begin to form. These new grains nucleate and grow, consuming and replacing the old, deformed grains that were filled with stresses and dislocations. This is the true "reset" of the material's microstructure.
Stage 3: Grain Growth
If the material is held at temperature for too long after recrystallization is complete, the new grains will continue to grow by merging with one another. This grain growth further reduces the material's hardness and strength. Controlling this stage is key to achieving the desired final properties.
Understanding the Key Trade-offs
While powerful, annealing is a process of balance. Misunderstanding its principles can lead to undesirable outcomes.
The Risk of Excessive Grain Growth
While some grain growth is inherent to the process, allowing it to become excessive can be detrimental. Overly large grains can significantly reduce the material's strength and toughness, even if it is very soft and ductile. The soaking time and temperature must be carefully controlled to prevent this.
The Critical Importance of Slow Cooling
The slow cooling rate is a defining feature of annealing. It allows the material's atoms to settle into their most stable, low-energy positions, ensuring maximum stress relief and softness. If the material were cooled quickly (a process known as quenching), it would lock in a much harder, more brittle structure—the exact opposite of annealing's goal.
Material-Specific Parameters
There is no universal annealing recipe. The ideal temperature and soaking time are highly dependent on the specific material and its alloy composition. Annealing steel requires very different parameters than annealing aluminum or brass.
How to Apply This to Your Project
Your decision to anneal should be driven by a clear engineering requirement.
- If your primary focus is preparing a material for forming or machining: Anneal to maximize ductility and softness, making the material easier to shape and cut with less risk of fracture.
- If your primary focus is reversing the effects of cold working: Use annealing to relieve internal stresses and restore the original, more ductile microstructure of the material.
- If your primary focus is improving electrical conductivity: For a material like copper, annealing repairs lattice defects that impede electron flow, thereby increasing its conductivity.
By understanding annealing, you gain precise control over a material's fundamental properties to meet your engineering goals.
Summary Table:
| Annealing Stage | Key Process | Resulting Material Change |
|---|---|---|
| Recovery | Dislocations move and reorganize. | Internal stresses are relieved. |
| Recrystallization | New, strain-free grains form. | Hardness decreases; ductility increases. |
| Grain Growth | New grains merge and grow. | Material becomes softer and more workable. |
Need precise thermal processing for your materials? The annealing process requires exact temperature control to achieve the desired material properties. KINTEK specializes in high-quality lab furnaces and ovens that deliver the uniform heating and precise soaking times essential for successful annealing. Whether you're working with steel, aluminum, or copper, our equipment helps you achieve optimal softness, ductility, and stress relief. Contact our experts today to find the perfect annealing solution for your laboratory's needs.
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
People Also Ask
- Why do industrial high-temperature diffusion furnaces require precise temperature control? Essential for Engine Blades
- What is the most commonly used filler metal in brazing? Discover the Best Alloys for Strong Joints
- Why is a vacuum oven necessary for pre-treating PBS and BP? Ensure Composite Integrity via Advanced Dehydration
- What are arc furnaces mainly used for? Efficiently Recycling Scrap into High-Quality Steel
- How does a sintering furnace influence EDC powder metallurgy electrodes? Optimize Your Tool for Superior Coatings
- How does the cooling rate control of a furnace influence slow-cooled solid-state electrolytes? Achieve Crystal Perfection
- Why is 1177 °C precision critical for GH3535 furnace treatment? Ensure Microstructural Integrity
- What does sintering do in powder metallurgy? Transform Powder into Strong, Solid Parts



















