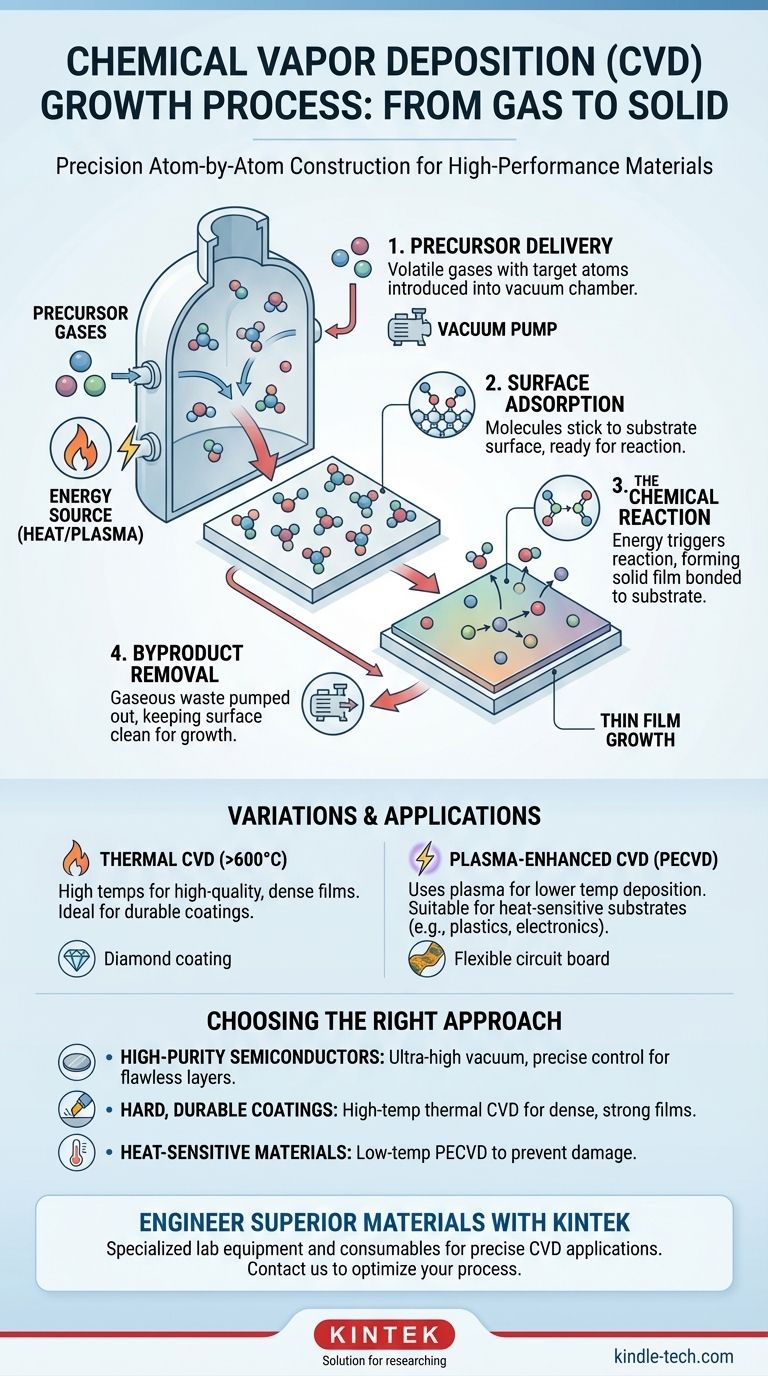
At its core, the chemical vapor deposition (CVD) growth process is a method for depositing a thin, solid film onto a surface, known as a substrate. This is achieved by placing the substrate inside a reaction chamber, introducing specific chemical precursor gases, and applying energy—typically heat—to trigger a chemical reaction that forms the desired material directly on the substrate's surface.
Chemical vapor deposition is not merely a coating technique; it is a precise, atom-by-atom construction process. It allows for the creation of exceptionally pure, high-performance materials by building them from a gaseous state, one chemical reaction at a time.

The Fundamental Goal: Building Materials from Gas
The primary purpose of CVD is to grow solid materials with highly controlled properties. Unlike melting and casting a material, CVD builds the material from the ground up.
This bottom-up approach provides incredible control over the final product's thickness, purity, and crystalline structure. It is the go-to method for applications where material performance is paramount.
Deconstructing the CVD Process: A Step-by-Step Breakdown
While different CVD methods exist, they all follow a similar sequence of events at the microscopic level. Understanding these steps is key to controlling the final outcome.
Step 1: Precursor Delivery
Volatile precursor gases, which contain the atoms needed for the final film, are injected into the reaction chamber. The chamber is typically under a vacuum to remove impurities and control the flow of these gases toward the substrate.
Step 2: Surface Adsorption
Once the precursor gas molecules reach the substrate, they stick to the surface in a process called adsorption. This is a necessary prelude to the chemical reaction.
Step 3: The Chemical Reaction
With the precursor molecules on the surface, energy (like heat) causes them to decompose or react with other gases. This reaction forms the desired solid material, which bonds directly to the substrate.
Over time, this process repeats, building up the thin film layer by layer. The nonvolatile products of the reaction are what form the film.
Step 4: Byproduct Removal
The chemical reaction also creates gaseous byproducts that are no longer needed. These waste products desorb (detach) from the surface and are transported away by the vacuum system, keeping the growth surface clean for new precursors to arrive.
Understanding the Trade-offs and Variations
CVD is not a single technique but a family of processes, each with its own advantages and limitations. The choice of method depends entirely on the desired material and the substrate being used.
Thermal CVD vs. Plasma-Enhanced CVD (PECVD)
Thermal CVD is the classic method, relying solely on high temperatures (often >600°C) to initiate the reaction. This produces very high-quality, dense films.
However, such high temperatures can damage sensitive substrates like plastics or certain electronic components. Plasma-Enhanced CVD (PECVD) solves this by using an electrical field to create a plasma, which provides the energy for the reaction. This allows deposition to occur at much lower temperatures.
The Challenge of Uniformity
A key goal of CVD is to create a film of perfectly even thickness across the entire substrate. While CVD is excellent at this, achieving perfect uniformity on complex, three-dimensional shapes can be a significant engineering challenge that requires careful control of gas flow and temperature gradients.
The Cost and Complexity
CVD systems, especially those for high-purity electronics, require sophisticated vacuum chambers, gas handling systems, and temperature controls. This makes the initial investment and operational complexity higher than simpler coating methods like painting or electroplating.
Making the Right Choice for Your Goal
Selecting the correct CVD approach requires aligning the process parameters with the application's specific needs.
- If your primary focus is high-purity semiconductors: You must prioritize ultra-high vacuum conditions and precisely controlled precursor gases to create flawless crystalline layers.
- If your primary focus is creating hard, durable coatings (like synthetic diamond): You should lean towards high-temperature thermal CVD variants to promote the growth of a dense, strongly bonded film.
- If your primary focus is coating heat-sensitive materials: You must use a low-temperature method like Plasma-Enhanced CVD (PECVD) to deposit the film without damaging the underlying substrate.
By mastering these principles, you can leverage chemical vapor deposition to engineer materials with properties tailored to nearly any advanced application.
Summary Table:
| Key CVD Process Step | What Happens | Key Outcome |
|---|---|---|
| Precursor Delivery | Gases containing target atoms are introduced into a vacuum chamber. | Precursors are delivered to the substrate surface. |
| Surface Adsorption | Gas molecules stick (adsorb) onto the substrate surface. | Precursors are in position for the reaction. |
| Chemical Reaction | Energy (heat/plasma) triggers a reaction, forming a solid material. | The desired thin film bonds to the substrate. |
| Byproduct Removal | Gaseous waste products are pumped away from the chamber. | A clean surface is maintained for continued growth. |
Ready to Engineer Superior Materials with CVD?
Mastering the Chemical Vapor Deposition process is key to developing next-generation semiconductors, durable protective coatings, and high-purity materials. The right lab equipment is critical for achieving the precise control, uniformity, and purity your research demands.
KINTEK specializes in providing the advanced lab equipment and consumables you need to excel. Whether you are developing electronics, creating hard coatings, or working with heat-sensitive substrates, we have the expertise and solutions to support your specific CVD applications.
Contact us today to discuss how our products can help you optimize your CVD process and achieve breakthrough results.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What role does CVD equipment play in single-layer graphene-coated metal catalysts? Unlock High-Performance Stability
- What is chemical Vapour deposition method for CNT? Achieve Precise Control for High-Quality Nanotube Growth
- What is the thermal chemical vapor deposition method for carbon nanotubes? The Key to Scalable Nanotube Production
- What is meant by vapor deposition? A Guide to Atomic-Level Coating Technology
- What are the parameters for chemical vapour deposition? Master Temperature, Pressure & Gas Flow for Perfect Films
- What are the potential applications of graphene? Unlocking Next-Gen Electronics, Energy, and Materials
- What is thin films explain sputtering techniques of thin film deposition? A Guide to DC, RF & Reactive Sputtering
- What is the process of ion beam sputtering? Achieve Superior Thin-Film Quality for High-Precision Applications



















