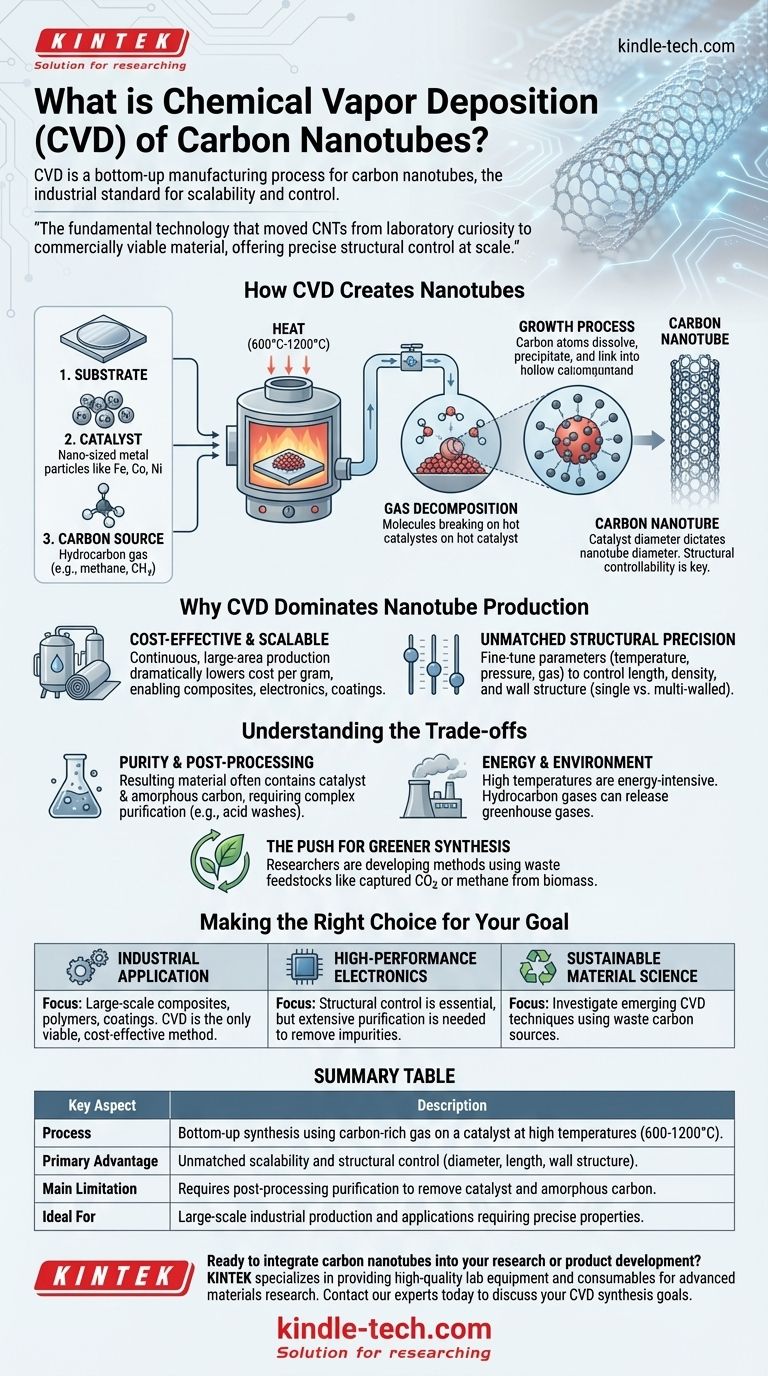
At its core, chemical vapor deposition (CVD) for carbon nanotubes is a bottom-up manufacturing process where a carbon-rich gas is heated in a furnace. This gas decomposes on tiny metallic catalyst particles, and the released carbon atoms self-assemble into hollow, cylindrical nanotube structures. This method has become the industrial standard because it is far more scalable and controllable than older techniques like arc discharge or laser ablation.
Chemical vapor deposition isn't just one way to make carbon nanotubes; it is the fundamental technology that moved them from a laboratory curiosity to a commercially viable material. Its value lies in offering precise structural control at a scale and cost that no other method can match.

How Chemical Vapor Deposition Creates Nanotubes
At a high level, the process is analogous to steam condensing into water on a cold surface. In CVD, however, a chemical reaction deposits solid carbon atoms from a gas.
The Core Components
The synthesis requires three key elements working in concert within a high-temperature reactor:
- The Substrate: This is the base material, typically silicon or quartz, that provides a stable surface for the reaction to occur.
- The Catalyst: These are nano-sized particles of metal, such as iron, cobalt, or nickel. The catalyst is the critical seed point where the nanotube growth begins.
- The Carbon Source: This is a hydrocarbon gas (like methane, acetylene, or ethylene) that flows into the reactor. It serves as the "feedstock" of carbon atoms.
The Growth Process: A Thermal Reaction
The process unfolds in a controlled sequence. First, the substrate, coated with catalyst nanoparticles, is heated to a high temperature, typically between 600°C and 1200°C.
Next, the carbon source gas is introduced into the reactor. The intense heat causes the gas molecules to break apart on the surface of the hot catalyst particles.
Finally, the now-liberated carbon atoms dissolve into and precipitate out of the catalyst particle, where they link together in the signature hexagonal pattern of a carbon nanotube, pushing upward like a blade of grass growing from the soil.
Why the Catalyst is the Key to Control
The process is more accurately called Catalytic Chemical Vapor Deposition (CCVD) because the catalyst is not just an initiator; it is a template.
The diameter of the catalyst nanoparticle directly dictates the diameter of the resulting nanotube. By carefully engineering the size of these catalyst particles before growth begins, manufacturers can produce nanotubes with specific, uniform diameters. This level of "structural controllability" is unique to CVD and is essential for creating materials with predictable electronic and mechanical properties.
Why CVD Dominates Nanotube Production
Older methods like laser ablation and arc discharge—which involve vaporizing pure graphite with intense energy—are effective for small-scale research but are impractical for mass production. CVD solved the problem of scale.
Cost-Effectiveness and Scalability
CVD is a more continuous and less energy-intensive process compared to its predecessors. It can be scaled to coat large-area substrates or run continuously in large reactors, dramatically lowering the cost per gram of nanotubes and enabling their use in composites, electronics, and coatings.
Unmatched Structural Precision
Beyond controlling diameter, operators can fine-tune other CVD parameters—such as temperature, pressure, and gas composition—to influence the length, density, and even the wall structure (single-walled vs. multi-walled) of the nanotubes. This makes CVD the go-to method for any application that requires tailored nanotube characteristics.
Understanding the Trade-offs
Despite its dominance, the CVD process is not perfect. Understanding its limitations is critical for practical applications.
Purity and Post-Processing
The resulting nanotube material is often a mixture containing not only the desired nanotubes but also leftover catalyst particles and undesirable, non-crystalline "amorphous carbon." These impurities must be removed through complex post-processing steps like acid washes, which adds cost and complexity.
Energy Consumption and Environmental Impact
The high temperatures required for CVD consume a significant amount of energy. Furthermore, the use of hydrocarbon feedstock gases means the process can release greenhouse gases. This synthesis step is the main source of the potential ecotoxicity and environmental footprint of carbon nanotubes.
The Push for Greener Synthesis
To address these environmental concerns, researchers are actively developing CVD methods that use "green" or waste feedstocks. This includes pioneering techniques to use captured carbon dioxide (CO2) or methane produced from biomass as the carbon source, aiming to make nanotube production more sustainable.
Making the Right Choice for Your Goal
Your application dictates how you should view the CVD process and its outputs.
- If your primary focus is large-scale industrial application: CVD is the only viable method for producing the necessary volume of CNTs for composites, polymers, or coatings in a cost-effective manner.
- If your primary focus is high-performance electronics: The structural control of CVD is essential, but you must factor in the need for extensive purification to remove metallic catalyst impurities that would otherwise disrupt device performance.
- If your primary focus is sustainable material science: Investigate emerging CVD techniques that utilize waste carbon sources like captured CO2 or pyrolyzed methane to align with environmental goals and reduce lifecycle impact.
Understanding the principles of CVD is the first step toward leveraging the remarkable properties of carbon nanotubes for future innovations.
Summary Table:
| Key Aspect | Description |
|---|---|
| Process | Bottom-up synthesis using carbon-rich gas on a catalyst at high temperatures (600-1200°C). |
| Primary Advantage | Unmatched scalability and structural control (diameter, length, wall structure) compared to older methods. |
| Main Limitation | Requires post-processing purification to remove catalyst particles and amorphous carbon impurities. |
| Ideal For | Large-scale industrial production (composites, coatings) and applications requiring precise nanotube properties. |
Ready to integrate carbon nanotubes into your research or product development?
The controlled synthesis of carbon nanotubes via CVD is key to unlocking their potential. KINTEK specializes in providing the high-quality lab equipment and consumables necessary for advanced materials research, including catalyst preparation and reactor systems.
Contact our experts today to discuss how our solutions can support your specific carbon nanotube synthesis goals, from initial research to scalable production.
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- Is chemical vapor deposition fast? Achieve High-Quality, Uniform Coatings at Industrial Pace
- What is thin film deposition in nanotechnology? Precision Engineering at the Atomic Scale
- What are the advantages of CVD for lithium anodes? Enhance Battery Stability with Precision Thin-Film Protection
- What is the gas deposition technique? A Guide to PVD and CVD Thin-Film Methods
- What is CVD method for graphene? A Scalable Process for High-Quality, Large-Area Films
- How does CVD work graphene? A Guide to Large-Scale, High-Quality Production
- What is the function of the external reaction generator in a CVD aluminizing system? Achieve Precision Coating Control
- What is the pressure range of LPCVD? Master the Key to Superior Film Conformality



















