The most common examples of gases that undergo deposition are water vapor, iodine vapor, and the gaseous forms of naphthalene and ammonium chloride. Deposition is the physical process where a gas turns directly into a solid, bypassing the liquid phase entirely. The most familiar real-world example of this is the formation of frost from water vapor on a cold morning.
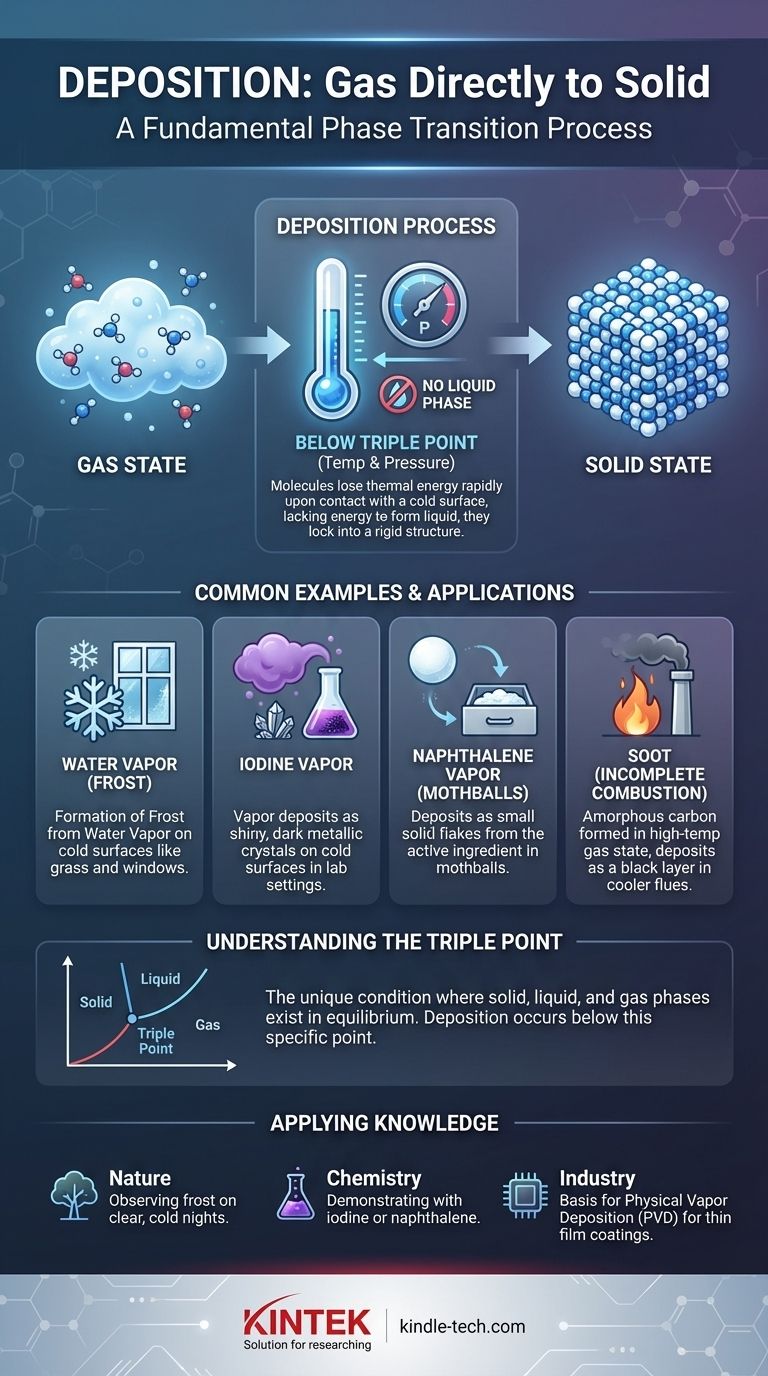
Deposition is not a property of a specific type of gas but rather a phase transition process. Any substance in a gaseous state can deposit into a solid if its temperature and pressure fall below a specific threshold known as the triple point.

What is Deposition? A Phase Transition Explained
Deposition is a fundamental thermodynamic process. It is the direct opposite of sublimation, where a solid turns directly into a gas.
From Gas Directly to Solid
When molecules in a gas lose thermal energy rapidly upon contact with a cold surface, they may lack sufficient energy to form a liquid. Instead, they lock directly into a rigid, crystalline structure, forming a solid.
The Role of Temperature and Pressure
This process is governed by a substance's phase diagram. For deposition to occur, the gas must be cooled to a temperature below its freezing point while its pressure is also below its triple point—the unique condition where the solid, liquid, and gas phases can all exist in equilibrium.
An Analogy: Frost on a Window
Think of a cold winter day. The air contains invisible water vapor (a gas). When this vapor touches a windowpane that is below freezing (0°C or 32°F), it doesn't condense into water droplets first; it instantly turns into delicate ice crystals. That is deposition in action.
Common Examples of Deposition
While any gas can theoretically deposit, some substances demonstrate this process under more familiar conditions.
Water Vapor to Ice (Frost)
This is the most prevalent example in nature. Frost on grass, car windshields, and other surfaces is not frozen dew. It is water vapor from the air that has deposited directly into solid ice.
Iodine Vapor to Crystalline Iodine
In a chemistry laboratory, gently heating solid iodine causes it to sublime into a vibrant purple vapor. When this vapor hits a cold surface, such as a watch glass with ice on it, it instantly deposits back into shiny, dark metallic crystals.
Naphthalene Vapor to Solid Flakes
Naphthalene is the active ingredient in traditional mothballs. The solid mothball slowly sublimes into a gas, and this gas can then deposit as small flakes in cooler, undisturbed parts of a drawer or closet.
Soot from Incomplete Combustion
Soot, which is primarily amorphous carbon, is formed in a high-temperature gaseous state during combustion. As it travels up a cooler chimney flue, it deposits as a solid black layer.
Why Some Substances are Better Examples
Not all gases seem to deposit as readily as water vapor or iodine. The reason lies in the conditions required for the transition.
The Importance of the Triple Point
Every substance has a unique triple point pressure. Deposition happens when a gas is at a pressure below this point.
For water, the triple point pressure is very low (about 0.006 atmospheres). This means anytime the temperature is below freezing and the air isn't saturated, deposition (frost) is possible.
Ease of Observation
Substances like iodine and naphthalene are classic examples because their triple point pressures are relatively high. This makes it easy to observe their sublimation and deposition cycles at or near standard atmospheric pressure in a simple lab setting.
In contrast, carbon dioxide's triple point is at over 5 atmospheres of pressure. This is why we see solid CO₂ (dry ice) sublime into a gas, but we do not see CO₂ gas deposit back into a solid under normal atmospheric conditions.
Applying This Knowledge
Understanding deposition is about recognizing the conditions, not memorizing a list of special gases.
- If your primary focus is observing this in nature: Pay attention to how frost forms on cold, clear nights, which is water vapor turning directly into ice.
- If your primary focus is chemistry: Understand that iodine and naphthalene are used as textbook examples because their phase transitions are easily demonstrated in a lab.
- If your primary focus is industrial applications: Recognize that this principle is the basis for Physical Vapor Deposition (PVD), a critical technology for applying thin film coatings in electronics and manufacturing.
Ultimately, deposition is a universal process that illustrates the direct relationship between a substance's state of matter and its energy.
Summary Table:
| Common Deposition Gas Examples | Typical Use Case | Key Characteristic |
|---|---|---|
| Water Vapor | Natural frost formation | Most common example in nature |
| Iodine Vapor | Chemistry lab demonstrations | Forms dark metallic crystals |
| Naphthalene Vapor | Mothball sublimation | Deposits as solid flakes |
| Carbon-based Gases | Soot formation in combustion | Industrial deposition process |
Need precise gas handling equipment for your deposition experiments? KINTEK specializes in laboratory equipment and consumables for phase transition studies and material science applications. Our reliable solutions ensure accurate temperature control and gas management for your research needs. Contact our experts today to discuss how we can support your laboratory's deposition and material analysis workflows!
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- CVD Diamond Domes for Industrial and Scientific Applications
People Also Ask
- Is sputtering a CVD? Understanding the Key Differences Between PVD and CVD
- What are the advantages and disadvantages of ALD? Precision vs. Speed in Thin Film Deposition
- How thick is chemical vapor deposition? Achieve Precise Control from Nanometers to Micrometers
- What are the limitations of ALD? Slow Deposition Speed and Material Constraints
- What is the temperature of a CVD furnace? From 200°C to 1600°C for Precise Film Deposition
- How does chemical deposition work? A Guide to Conformal Thin-Film Coating
- What is the general process of growing diamonds using the CVD method? Master Precision Lab-Grown Diamond Technology
- What are the advantages of maintaining a low reaction pressure (2000 Pa) for BDD films? Unlock Precision Nucleation



















