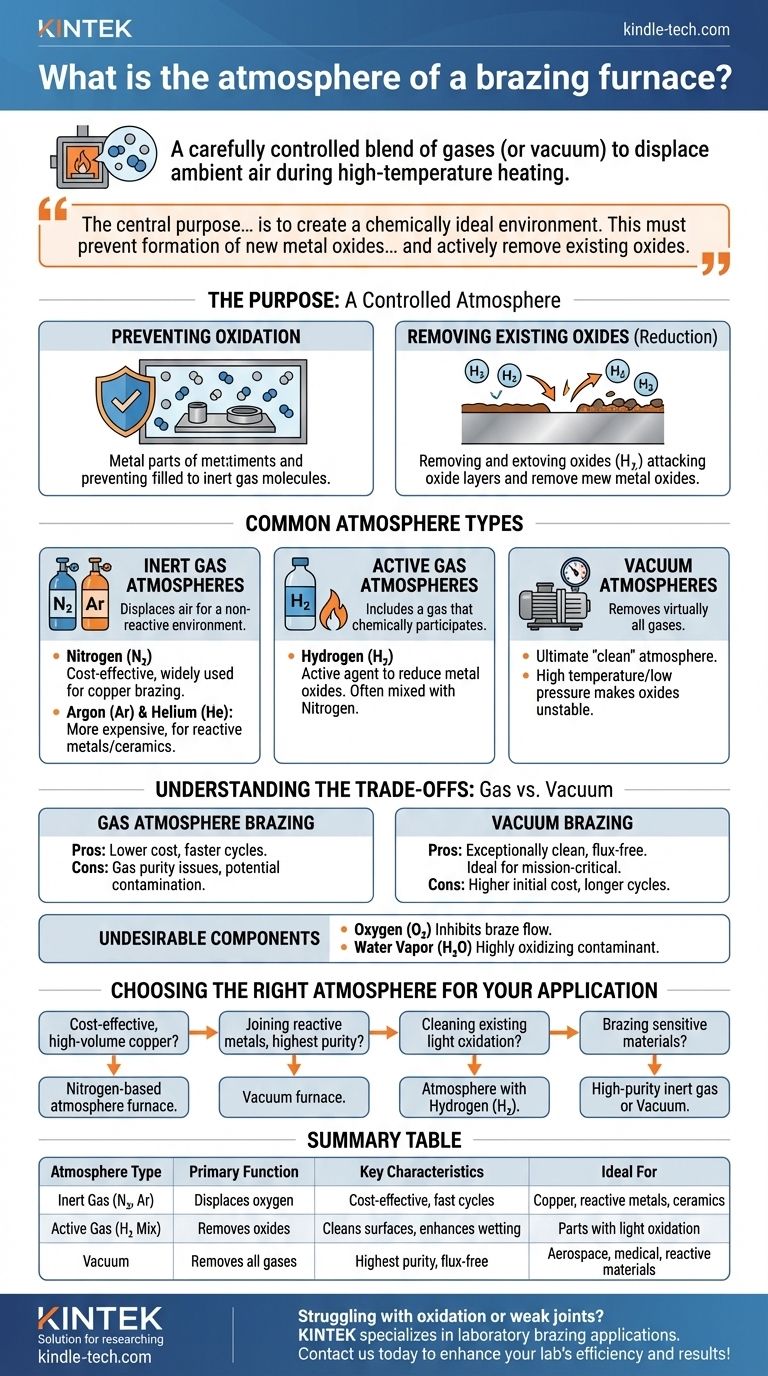
In brazing, the furnace "atmosphere" is the carefully controlled blend of gases—or the lack thereof in a vacuum—that surrounds the parts during the high-temperature heating cycle. This environment is actively managed to displace ambient air, primarily oxygen, which would otherwise ruin the brazing process by creating oxide layers on the metal surfaces that prevent the filler metal from bonding.
The central purpose of a brazing furnace atmosphere is to create a chemically ideal environment. This environment must not only prevent the formation of new metal oxides at high temperatures but also, in many cases, actively remove existing oxides to ensure the braze filler metal can properly wet and bond to the base materials.

The Purpose of a Controlled Atmosphere
When metals are heated to brazing temperatures, they react aggressively with oxygen in the air. This reaction, called oxidation, creates a film on the surface of the parts. A controlled atmosphere is the solution to this fundamental problem.
Preventing Oxidation
The most basic function of a brazing atmosphere is to displace oxygen. By filling the furnace chamber with a specific gas like nitrogen or argon, or by removing the air to create a vacuum, you eliminate the oxygen that would otherwise cause damaging oxidation.
Removing Existing Oxides (Reduction)
A more advanced function is to actively clean the parts. Atmospheres containing an active gas, most commonly hydrogen (H₂), can chemically react with and remove light oxides that were already on the metal surfaces before they entered the furnace. This process is known as reduction.
Common Types of Brazing Atmospheres
The choice of atmosphere depends on the materials being joined, the required joint quality, and cost considerations. The primary methods involve using specific gases or creating a vacuum.
Inert Gas Atmospheres
Inert gases are used to displace air and provide a neutral, non-reactive environment. A slight vacuum is often pulled first to remove the bulk of the air before the chamber is backfilled with the inert gas.
- Nitrogen (N₂): A cost-effective and widely used atmosphere, especially for brazing copper and its alloys. It is excellent at displacing oxygen.
- Argon (Ar) & Helium (He): These are more expensive inert gases used for brazing reactive metals (like titanium) or ceramics that might react negatively with nitrogen.
Active Gas Atmospheres
These atmospheres include a gas that actively participates in the process.
- Hydrogen (H₂): As the primary active agent, hydrogen is invaluable for its ability to reduce metal oxides. It is often mixed in small percentages with nitrogen to create a reducing atmosphere that cleans the parts as they are heated.
Vacuum Atmospheres
A vacuum furnace doesn't introduce a gas; instead, it removes virtually all gases from the heating chamber using powerful pumps. This is the ultimate "clean" atmosphere.
At the very low pressures and high temperatures inside a vacuum furnace, many metal oxides become unstable and simply break down or "sublimate" off the part surface. This leaves an exceptionally clean base metal for the braze filler to flow over.
Understanding the Trade-offs: Gas vs. Vacuum
Both gas and vacuum atmospheres are effective, but they serve different needs and come with distinct advantages and disadvantages.
Gas Atmosphere Brazing
This method offers great flexibility and is highly efficient for many common applications. The furnace is sealed and purged with a controlled gas mixture.
- Pros: Generally lower equipment cost, faster cycle times than vacuum, and highly effective for materials like copper when using a nitrogen-based atmosphere.
- Cons: Requires careful management of gas purity and flow rates. The presence of impurities like water vapor or residual oxygen can still cause oxidation.
Vacuum Brazing
Considered the premium brazing process, vacuum brazing excels in high-purity and mission-critical applications.
- Pros: Produces exceptionally clean, strong, and flux-free joints. It is ideal for complex geometries and reactive materials found in aerospace, medical, and scientific fields.
- Cons: Higher initial equipment cost and potentially longer cycle times due to the need to pump down the chamber to a deep vacuum.
Undesirable Components
Regardless of the method, two components are almost always detrimental to the process:
- Oxygen (O₂): The primary cause of oxidation that inhibits braze flow.
- Water Vapor (H₂O): Can be highly oxidizing at brazing temperatures and is generally considered an undesirable contaminant in the furnace atmosphere.
Choosing the Right Atmosphere for Your Application
Selecting the correct atmosphere is critical for achieving a successful, reliable braze joint. Your choice should be driven by the material and the end-use requirements of the part.
- If your primary focus is cost-effective, high-volume copper brazing: A nitrogen-based atmosphere furnace is an excellent and efficient choice.
- If your primary focus is joining reactive metals or achieving the highest-purity joints for aerospace: A vacuum furnace is the superior solution, as it eliminates flux and ensures maximum joint integrity.
- If your primary focus is cleaning parts with existing light oxidation during the process: An atmosphere containing a percentage of hydrogen (H₂) will act as a reducing agent to prepare the surface.
- If your primary focus is brazing sensitive materials like aluminum or certain ceramics: A high-purity inert gas atmosphere (like argon) or a vacuum furnace will provide the necessary non-reactive environment.
Ultimately, controlling the furnace atmosphere is about creating the ideal chemical environment for the filler metal to bond perfectly with the base metals.
Summary Table:
| Atmosphere Type | Primary Function | Key Characteristics | Ideal For |
|---|---|---|---|
| Inert Gas (N₂, Ar) | Displaces oxygen to prevent oxidation | Cost-effective, fast cycle times | Copper, reactive metals, ceramics |
| Active Gas (H₂ Mix) | Removes existing oxides via reduction | Cleans surfaces, enhances wetting | Parts with light oxidation |
| Vacuum | Removes all gases; oxides break down at high heat | Highest purity, flux-free joints | Aerospace, medical, reactive materials |
Struggling with oxidation or weak joints in your brazing process? KINTEK specializes in lab equipment and consumables, providing tailored solutions for laboratory brazing applications. Our expertise ensures you achieve the perfect atmosphere control for strong, reliable metal joints. Contact us today to discuss how our brazing furnaces and consumables can enhance your lab's efficiency and results!
Visual Guide
Related Products
- Horizontal High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Furnace Negative Material Graphitization Furnace
- Vacuum Heat Treat and Pressure Sintering Furnace for High Temperature Applications
- Graphite Vacuum Furnace Bottom Discharge Graphitization Furnace for Carbon Materials
- 1200℃ Muffle Furnace Oven for Laboratory
People Also Ask
- Why is a 1000°C+ furnace needed for LLZO/LLTO? Mastering High-Temperature Sintering for Ceramic Electrolytes
- What is the function of a high-temperature furnace during burnout? Master Aluminum Foam Production with Precision
- Why is a high-temperature furnace with atmosphere control required for rGO? Enhance Your Carbon Research Quality
- Why is a high-temperature furnace required for Li7P2S8I synthesis? Unlock Peak Ionic Conductivity
- Why is a high-vacuum graphite heating element furnace used for HAp sintering? Achieve Pure, High-Bond Coatings













