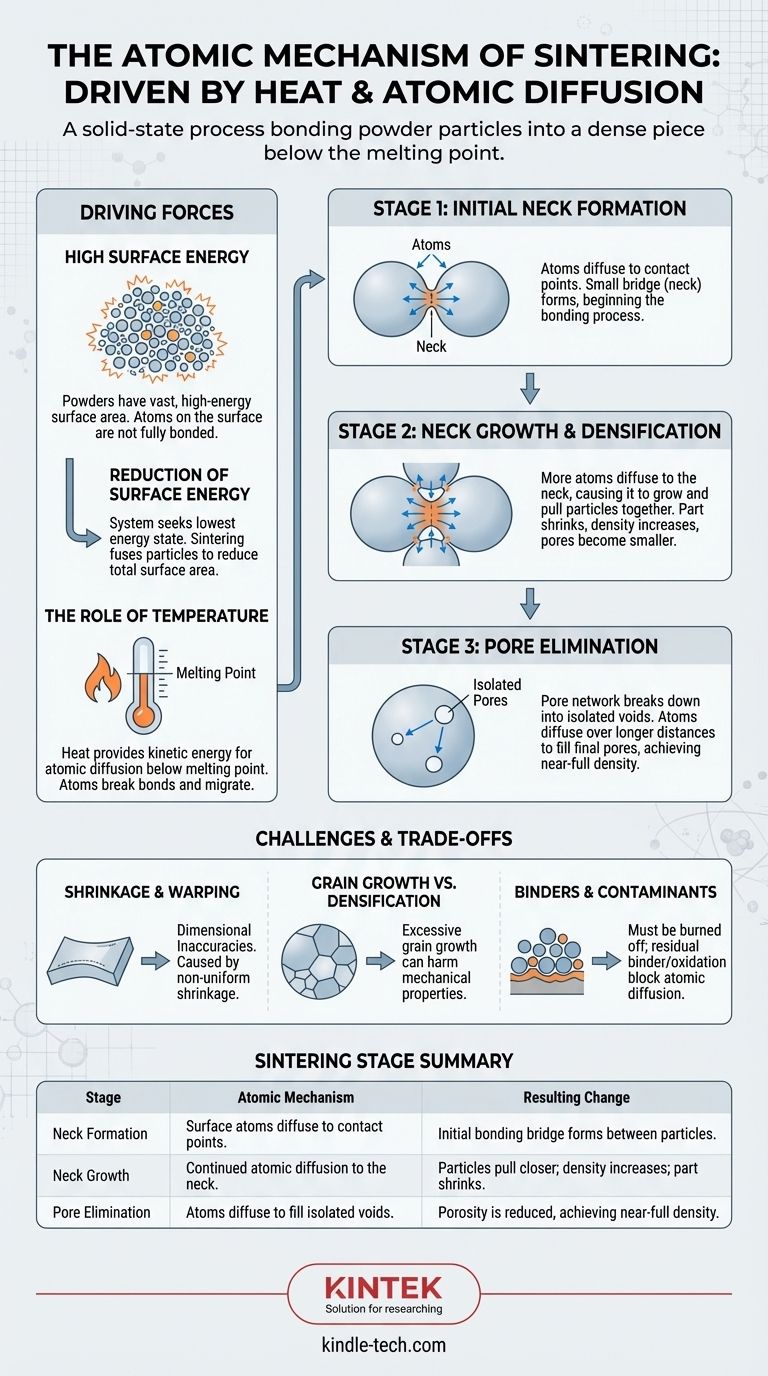
At its most fundamental level, sintering is the process of atomic diffusion driven by heat. Individual powder particles, when heated below their melting point, have atoms that migrate across their boundaries. This migration closes the gaps between particles, bonding them together to form a dense, solid piece.
The core principle of sintering is the reduction of surface energy. A fine powder has a vast amount of high-energy surface area, and by using thermal energy, atoms rearrange themselves to eliminate these surfaces and the voids between them, resulting in a more stable, lower-energy solid structure.

The Driving Force: Why Sintering Happens
Sintering is not melting; it is a solid-state transformation. The process is governed by fundamental thermodynamic principles that push the material toward a more stable state.
High Surface Energy of Powders
A collection of fine powder has an enormous amount of surface area relative to its volume. Surfaces represent a state of higher energy because atoms at a surface are not fully bonded like those in the bulk material.
The system naturally seeks its lowest possible energy state. Sintering provides a pathway for the powder compact to reduce its total surface area by fusing the particles together.
The Role of Temperature
Heat provides the necessary kinetic energy for atoms to break their existing bonds and move. This movement, or diffusion, is the engine of sintering.
Crucially, the temperature is kept below the material's melting point. The goal is to make the atoms mobile enough to rearrange themselves, not to turn the entire mass into a liquid.
The Stages of Atomic Diffusion
The transformation from a loose powder to a dense solid occurs in distinct, overlapping stages, all governed by the movement of atoms.
Stage 1: Initial Neck Formation
When two particles touch, a small contact point exists. As heat is applied, atoms from the surface of the particles begin to diffuse to this contact point.
This migration of atoms forms a small bridge, or "neck," between the two particles. This is the very beginning of the bonding process.
Stage 2: Neck Growth and Densification
As sintering continues, more atoms diffuse to the neck, causing it to grow wider and stronger. This process pulls the centers of the adjacent particles closer together.
On a macroscopic scale, this collective pulling-together of millions of particles is what causes the entire part to shrink and its density to increase. The pores, or voids between particles, become smaller and more rounded.
Stage 3: Pore Elimination
In the final stage, the pore network has broken down into isolated, often spherical voids within the now-solid material.
The complete elimination of these final pores is a slow process, as atoms must diffuse over longer distances to fill them. Eventually, a nearly fully dense part can be achieved.
Understanding the Trade-offs and Challenges
While driven by a simple atomic mechanism, the sintering process involves a delicate balance of competing phenomena that present practical challenges.
Shrinkage and Warping
The same atomic movement that causes densification also causes the part to shrink. If this shrinkage is not uniform—due to gravity, friction with the furnace floor, or inconsistent powder packing—the part can warp or sag, leading to dimensional inaccuracies.
Grain Growth vs. Densification
The thermal energy that drives diffusion also drives grain growth, a process where larger crystal grains within the material consume smaller ones.
While densification is usually desirable, excessive grain growth can be detrimental to mechanical properties like strength and toughness. A successful sintering process maximizes density while controlling grain size.
The Role of Binders and Contaminants
Most powder compacts (known as "green bodies") contain organic binders for handling strength. These must be completely burned off at lower temperatures before sintering begins.
Any residual binders or surface oxidation on the powder particles can act as a barrier, physically blocking atom-to-atom contact and inhibiting the diffusion necessary for bonding.
How Different Techniques Leverage This Mechanism
Different sintering methods control the process by manipulating the driving forces of heat and pressure to influence atomic diffusion.
Conventional (Pressureless) Sintering
This is the simplest form, relying solely on thermal energy to drive atomic diffusion. The powder compact is simply heated in a furnace until the desired density is reached.
Pressure-Assisted Sintering
Techniques like Hot Pressing apply external pressure during heating. This mechanical force pushes particles together, enhancing contact and accelerating the diffusion and densification process. It often allows for lower temperatures or shorter cycle times.
Liquid Phase Sintering (LPS)
In this technique, a small amount of an additive is used which melts into a liquid at the sintering temperature. This liquid wets the solid particles and acts as a rapid transport path for atoms, which dissolve into the liquid and re-precipitate at the necks, dramatically accelerating densification.
Direct Metal Laser Sintering (DMLS)
Used in metal 3D printing, DMLS uses a high-power laser to provide extremely localized and rapid heating. It fuses the metal powder layer by layer, with the laser's energy driving the atomic diffusion and bonding almost instantaneously in a very small area.
Making the Right Choice for Your Goal
The optimal sintering strategy depends entirely on the desired properties of the final component.
- If your primary focus is maximum density and mechanical properties: Pressure-assisted methods or Liquid Phase Sintering are superior, as they are more effective at eliminating porosity and controlling grain structure.
- If your primary focus is cost-effectiveness for simple shapes: Conventional pressureless sintering is often the most economical choice and is sufficient for many applications.
- If your primary focus is producing complex, near-net-shape parts: Additive manufacturing techniques like DMLS are ideal, as they build the final shape directly by fusing powder layer by layer.
Understanding this atomic-level dance of diffusion is the key to mastering the sintering process and engineering materials with intention.
Summary Table:
| Sintering Stage | Atomic Mechanism | Resulting Change |
|---|---|---|
| Neck Formation | Surface atoms diffuse to contact points. | Initial bonding bridge forms between particles. |
| Neck Growth | Continued atomic diffusion to the neck. | Particles pull closer; density increases; part shrinks. |
| Pore Elimination | Atoms diffuse to fill isolated voids. | Porosity is reduced, achieving near-full density. |
Master the Sintering Process for Your Laboratory Needs
Understanding the atomic mechanism of sintering is the first step to optimizing your materials processing. Whether you are developing new ceramics, metals, or advanced composites, precise control over temperature, pressure, and atmosphere is critical for achieving the desired density, strength, and microstructure in your final components.
KINTEK is your partner in precision sintering. We specialize in supplying high-quality lab furnaces, presses, and consumables tailored for research and production. Our equipment helps you:
- Achieve superior densification with precise temperature control.
- Minimize warping and control grain growth for consistent, high-integrity parts.
- Explore various techniques, from conventional to liquid phase sintering, with the right tools.
Ready to enhance your sintering capabilities? Let our experts help you select the ideal equipment for your specific material and application goals.
Contact KINTEL today for a consultation and let's engineer your material's success together.
Visual Guide
Related Products
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Spark Plasma Sintering Furnace SPS Furnace
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- Why is sintering easier in the presence of a liquid phase? Unlock Faster, Lower-Temperature Densification
- What is the standard thickness of plating? Optimize Durability, Corrosion & Cost
- Why is a high vacuum environment necessary in sintering equipment for TiAl alloys? Ensure High-Purity Metal Bonding
- What is sintering reaction? Transform Powders into Dense Solids Without Melting
- How does precise temperature control affect FeCoCrNiMnTiC high-entropy alloys? Master Microstructural Evolution



















