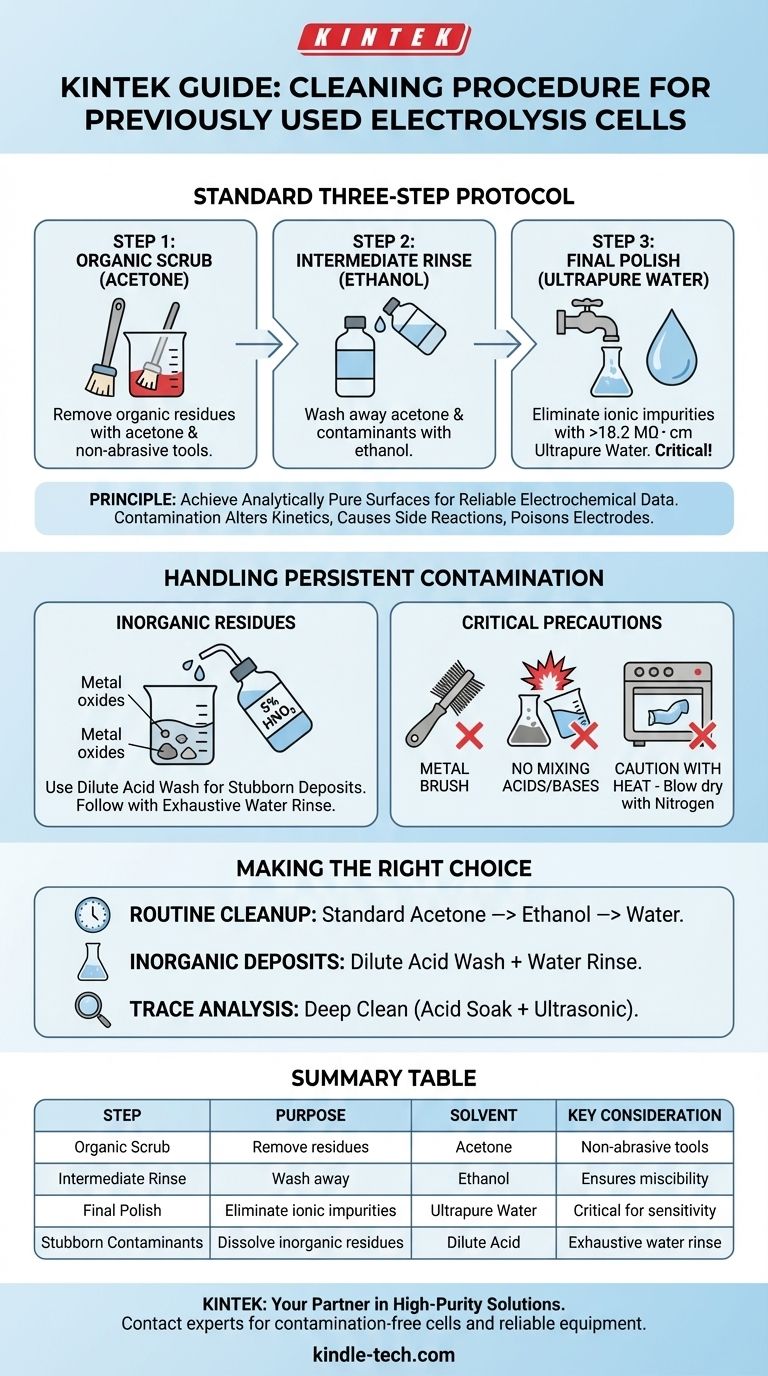
The standard procedure for cleaning a previously used electrolysis cell involves a sequential three-step rinse. First, scrub the inner walls with acetone to remove organic residues, then rinse thoroughly with ethanol, and finish with a final, meticulous rinse using ultrapure water with a resistivity greater than 18.2 MΩ・cm.
The goal of any cleaning protocol is not just to make the cell look clean, but to achieve an analytically pure surface. The specific solvents and methods you use must be chosen to systematically remove all potential contaminants from previous experiments without damaging the cell itself.

The Principle: Why Meticulous Cleaning is Non-Negotiable
In electrochemistry, even trace amounts of contamination can alter reaction kinetics, introduce unwanted side reactions, or poison electrode surfaces. This invalidates your data.
A disciplined cleaning regimen ensures that the results you measure are a true reflection of the chemical system you intend to study, not an artifact of leftover residue.
The Standard Three-Step Protocol
This procedure is the workhorse for routine cleaning immediately after an experiment. It effectively removes a wide range of common organic contaminants and residual salts.
Step 1: The Organic Scrub (Acetone)
Acetone is an excellent solvent for a wide variety of non-polar and polar organic compounds. A gentle scrub with a non-abrasive wipe or brush soaked in acetone will dissolve and lift most organic residues left from reactants or solvents.
Step 2: The Intermediate Rinse (Ethanol)
Ethanol is miscible with both acetone and water. Its primary role is to thoroughly wash away the acetone and any dissolved contaminants, preparing the surface for the final, critical rinse.
Step 3: The Final Polish (Ultrapure Water)
This is the most critical step. Rinsing with ultrapure water (Type I, with a resistivity of 18.2 MΩ・cm) removes any remaining ethanol and, most importantly, any trace ionic impurities that could interfere with your electrochemical measurements. Standard deionized water is often insufficient for sensitive work.
Handling More Persistent Contamination
Sometimes, the standard protocol isn't enough, especially if residues have dried or are inorganic in nature.
Using Dilute Acids or Bases
For stubborn inorganic residues like metal oxides, a dilute acid wash may be necessary. Soaking the cell in a solution like 5% nitric acid (HNO₃) can effectively dissolve these contaminants. Similarly, a dilute base may be used for specific acidic residues.
The Importance of Thorough Rinsing
After any acid or base treatment, you must rinse the cell extensively with ultrapure water. Any residual acid or base will drastically alter the pH and conductivity of your next experiment.
Critical Precautions to Protect Your Equipment
Improper cleaning can be more destructive than no cleaning at all. Adhering to these safety and handling guidelines is essential for the longevity of your cell and the integrity of your research.
Avoid Abrasive Tools
Never use metal brushes or other hard abrasives. These tools will create microscopic scratches on the glass surface, which can trap contaminants and become impossible to clean properly.
Do Not Mix Cleaning Agents
A dangerous exothermic reaction can occur if you mix acids and bases, such as nitric acid and sodium hydroxide. Always handle and dispose of cleaning solutions separately and rinse thoroughly between steps.
Be Cautious with Heat
While the glass body can often be sterilized, the entire cell assembly should not be heated. Materials like PTFE (Teflon) can expand and deform permanently, while others like POM can crack under high heat. If drying is needed, blowing with pure nitrogen gas is a safer alternative to oven drying.
Making the Right Choice for Your Goal
Select your cleaning procedure based on the previous experiment and the sensitivity of the next one.
- If your primary focus is routine post-experiment cleanup: The standard acetone -> ethanol -> ultrapure water sequence is your go-to protocol.
- If you suspect significant inorganic or metallic deposits: Use a dilute acid wash (e.g., HNO₃), followed by an exhaustive rinse with ultrapure water.
- If you are preparing for a highly sensitive trace analysis: Consider a deep clean involving an acid soak and ultrasonic cleaning to ensure a pristine baseline.
A consistent and appropriate cleaning protocol is the foundation of reliable and reproducible electrochemical data.
Summary Table:
| Step | Purpose | Recommended Solvent | Key Consideration |
|---|---|---|---|
| 1. Organic Scrub | Remove organic residues | Acetone | Use non-abrasive tools |
| 2. Intermediate Rinse | Wash away acetone and contaminants | Ethanol | Ensures miscibility |
| 3. Final Polish | Eliminate ionic impurities | Ultrapure Water (>18.2 MΩ・cm) | Critical for sensitive measurements |
| For Stubborn Contaminants | Dissolve inorganic residues | Dilute Acid (e.g., 5% HNO₃) | Follow with exhaustive water rinse |
Achieve Analytically Pure Surfaces with KINTEK
Reproducible electrochemical data starts with a contamination-free cell. KINTEK specializes in high-purity lab equipment and consumables, providing the reliable tools and expert support your laboratory needs to maintain impeccable standards.
Whether you require certified solvents, ultrapure water systems, or durable, easy-to-clean cell components, we have the solutions to enhance your protocol's effectiveness and protect your investment.
Ensure the integrity of your next experiment. Contact our experts today to discuss your specific cleaning challenges and how our products can help.
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What role do zirconia porous ceramics play in a supercritical fluid electrochemical cell? Ensure Data Integrity.
- What role does a three-electrode system electrolytic cell play in simulated corrosion environment testing?
- What is the overall structure of the H-type electrolytic cell? Understanding Dual-Chamber Electrochemical Designs
- What is the typical volume range for a single chamber of the H-type electrolytic cell? Find Your Ideal Lab Capacity
- What is the typical volume range for the five-port water bath electrolytic cell? From 10ml to 1000ml
- What is the purpose of an electrolytic etching system for 310H stainless steel? Reveal Precise Microstructure Details
- What is the general procedure for operating the in-situ Raman electrolytic cell? Master the 3-Phase Setup for Accurate Data
- How is electrochemical fragmentation used to increase liquid metal surface area? Boost Catalytic Efficiency



















