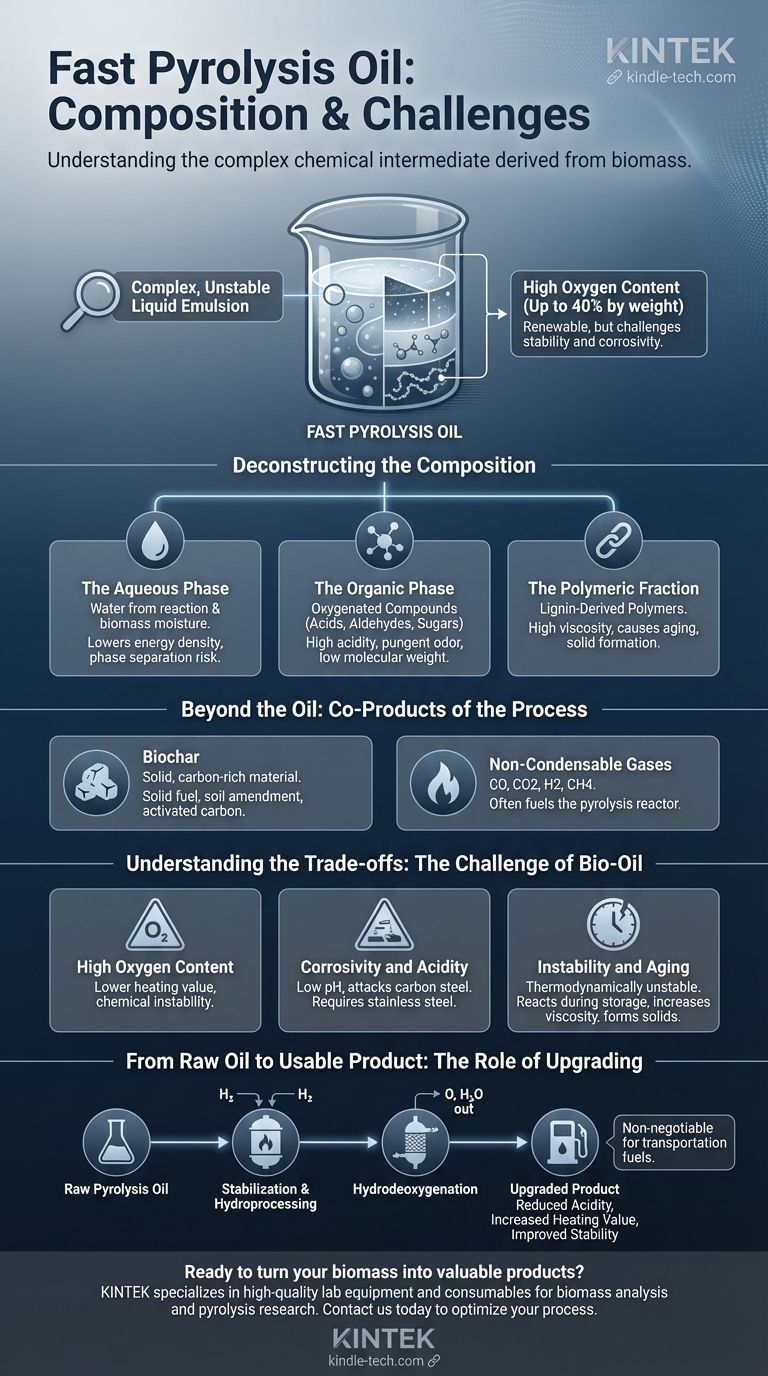
At its core, fast pyrolysis oil is a complex and unstable liquid emulsion. It consists primarily of a dense mixture of highly oxygenated organic compounds, water, and polymers derived directly from the thermal decomposition of biomass.
The defining characteristic of fast pyrolysis oil is its high oxygen content—up to 40% by weight. This makes it a promising renewable resource, but also presents significant challenges in terms of stability, corrosivity, and compatibility with conventional fuel systems.

Deconstructing the Composition
To effectively use or upgrade pyrolysis oil, you must first understand its distinct components. It is not a uniform substance but a micro-emulsion of an organic-rich phase and an aqueous phase.
The Aqueous Phase: Water Content
Water is a significant product of the pyrolysis reaction and is also present as moisture in the original biomass feedstock. It becomes thoroughly mixed into the final oil, contributing to its low energy density and potential for phase separation over time.
The Organic Phase: Oxygenated Compounds
This is the most complex part of the oil. It is a mixture of hundreds of different organic compounds, which can be grouped by molecular weight.
- Low Molecular Weight: These include acids (like acetic acid), aldehydes (like formaldehyde), and ketones. These compounds are largely responsible for the oil's high acidity and pungent odor.
- High Molecular Weight: These are primarily phenols and other aromatic compounds derived from lignin, as well as sugars and oligosaccharides from cellulose.
The Polymeric Fraction: Lignin-Derived Polymers
Pyrolysis oil also contains larger, non-volatile molecules often referred to as pyrolytic lignin or polymers. These compounds contribute significantly to the oil's high viscosity and tend to polymerize further during storage, causing the oil to age, thicken, and eventually form solids.
Beyond the Oil: Co-Products of the Process
Fast pyrolysis does not only produce oil. Understanding the other outputs is critical for evaluating the overall efficiency and economics of the system.
Biochar
This solid, carbon-rich material is the "charcoal" byproduct of pyrolysis. It can be used as a solid fuel, a soil amendment (biochar), or for producing activated carbon.
Non-Condensable Gases
The process also generates flammable gases that do not condense into the liquid oil. This mixture includes carbon monoxide (CO), carbon dioxide (CO2), hydrogen (H2), and methane (CH4). In a well-designed system, these gases are burned to provide the heat required for the pyrolysis reactor, making the process largely self-sustaining.
Understanding the Trade-offs: The Challenge of Bio-Oil
The unique composition of pyrolysis oil creates a clear set of technical hurdles that must be overcome for its widespread use. Its properties are fundamentally different from those of conventional hydrocarbon fuels.
High Oxygen Content
The high oxygen content is the root cause of most other issues. It leads to a lower heating value (typically 50-70% that of heavy fuel oil) and chemical instability.
Corrosivity and Acidity
The presence of acetic acid and other organic acids gives the oil a low pH, making it highly corrosive to common construction materials like carbon steel. This necessitates the use of more expensive stainless steel in storage tanks, pumps, and pipelines.
Instability and Aging
Pyrolysis oil is thermodynamically unstable. The reactive aldehydes, alcohols, and polymers can continue to react during storage, a process known as "aging." This increases the oil's viscosity, can lead to the formation of solids, and may cause it to separate into distinct aqueous and organic phases.
From Raw Oil to Usable Product: The Role of Upgrading
Due to these challenges, raw pyrolysis oil is rarely a drop-in replacement for conventional fuels. It typically requires upgrading to be used in engines or refineries.
Stabilization and Hydroprocessing
Upgrading techniques focus on removing oxygen through catalytic reactions with hydrogen, a process known as hydrodeoxygenation or hydroprocessing. This process reduces acidity, increases the heating value, and improves the oil's long-term stability, making it more compatible with existing fuel infrastructure.
Applying This to Your Goal
Your strategy for handling pyrolysis oil depends entirely on its intended application.
- If your primary focus is direct combustion in boilers or turbines: The key is to manage its corrosivity with appropriate materials and design burner systems that can handle its higher viscosity and lower energy content.
- If your primary focus is producing transportation fuels: Upgrading through hydroprocessing is non-negotiable to remove oxygen, reduce acidity, and create a stable, hydrocarbon-like product compatible with engines and refineries.
- If your primary focus is creating chemical commodities: The goal is to develop separation techniques to isolate high-value compounds like phenols or specific acids from the complex mixture, rather than using it as a bulk fuel.
Understanding that pyrolysis oil is not a single fuel but a complex chemical intermediate is the first step toward harnessing its potential.
Summary Table:
| Component | Description | Key Characteristics |
|---|---|---|
| Aqueous Phase | Water from reaction & biomass moisture | Lowers energy density, risk of phase separation |
| Organic Phase | Oxygenated compounds (acids, aldehydes, sugars) | High acidity, pungent odor, low molecular weight |
| Polymeric Fraction | Lignin-derived polymers (pyrolytic lignin) | High viscosity, causes aging and solid formation |
| Biochar (Co-product) | Solid, carbon-rich material | Used as fuel, soil amendment, or for activated carbon |
| Non-Condensable Gases (Co-product) | CO, CO2, H2, CH4 | Often used to fuel the pyrolysis reactor itself |
Ready to turn your biomass into valuable products?
Understanding the complex composition of pyrolysis oil is the first step. The next is selecting the right equipment for your specific application, whether it's direct combustion, fuel upgrading, or chemical extraction.
KINTEK specializes in high-quality lab equipment and consumables for biomass analysis and pyrolysis research. We can provide the tools you need to characterize your feedstock, optimize your process, and analyze your bio-oil effectively.
Contact us today to discuss how our solutions can help you overcome the challenges of pyrolysis oil and achieve your renewable energy or chemical production goals. Get in touch via our contact form.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Custom PTFE Teflon Parts Manufacturer PTFE Beaker and Lids
People Also Ask
- Is pyrolysis oil hazardous? The Critical Risks of Handling This Reactive Fuel
- What is the function of a rotary furnace? Achieve Uniform, Continuous Thermal Processing
- What are the steps of pyrolysis? A Complete Guide to the 3-Phase Process
- What are the methods of producing bio-oil? The Definitive Guide to Pyrolysis and Alternative Biofuel Processes
- What are the components of a pyrolysis machine? A Complete Breakdown of the Core System
- What are the different types of pyrolysis reactions? A Guide to Optimizing Biochar, Bio-Oil, and Syngas
- What is calcination suitable for? A Guide to High-Temperature Solid-State Transformation
- What is the difference between batch and continuous pyrolysis? Choose the Right System for Your Scale












