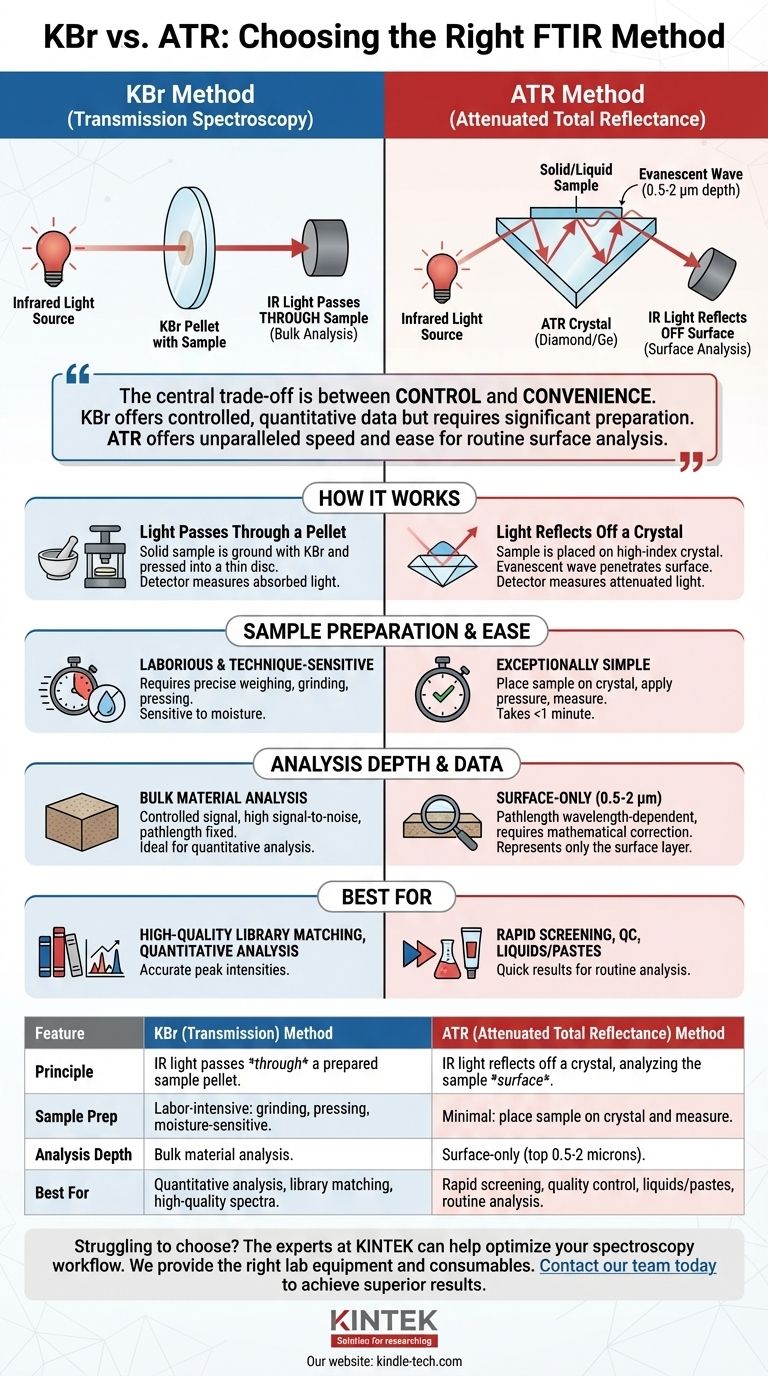
At its core, the difference between the KBr method and the ATR method is how the infrared (IR) light interacts with your sample. The KBr pellet method is a traditional transmission technique where IR light passes directly through a carefully prepared solid sample. In contrast, Attenuated Total Reflectance (ATR) is a surface technique where the IR light reflects off an internal crystal and only penetrates a few microns into a sample placed on top of it.
The central trade-off is between control and convenience. The KBr method offers highly controlled, quantitative spectral data but requires significant, moisture-sensitive sample preparation. ATR offers unparalleled speed and ease of use for routine analysis but provides information only about the sample's surface.

How Each Method Works
To choose the right technique, you must first understand the fundamental difference in how they generate a spectrum.
The KBr Pellet Method: Transmission Spectroscopy
In this classic method, a small amount of your solid sample is finely ground and intimately mixed with dry potassium bromide (KBr) powder. KBr is used because it is transparent to infrared radiation.
This mixture is then pressed under high pressure in a die to form a small, thin, transparent disc or "pellet." The IR beam of the spectrometer is directed straight through this pellet, and the detector measures the light that is absorbed by the sample at different wavelengths.
The ATR Method: Surface Reflection
ATR works on a completely different principle. An ATR accessory contains a high-refractive-index crystal, typically made of diamond or germanium.
The IR beam is directed into this crystal at a specific angle. The beam bounces, or internally reflects, off the top surface of the crystal where your sample is placed. At each reflection, an energy field called an evanescent wave extends a very short distance (typically 0.5 to 2 microns) beyond the crystal's surface and into the sample. The sample absorbs energy from this wave, and the attenuated (weakened) IR beam is then directed to the detector.
Key Differences in Application and Results
The practical implications of these two mechanisms directly impact your workflow and the type of data you can collect.
Sample Preparation and Ease of Use
This is the most significant practical difference. ATR is exceptionally simple. You place your solid or liquid sample directly onto the crystal, apply pressure to ensure good contact, and begin the measurement. The entire process can take less than a minute.
The KBr method is laborious and technique-sensitive. It requires precise weighing, extensive grinding to reduce particle size, and careful pressing to create a uniform pellet. The process is also highly susceptible to moisture contamination, as KBr is hygroscopic.
Control Over Signal Intensity
The KBr method gives you direct control over the signal strength. You can adjust the concentration of the sample within the KBr matrix or change the thickness (pathlength) of the pellet itself.
This control is a key advantage for quantitative analysis, where adherence to the Beer-Lambert law is critical.
Spectral Quality and Signal-to-Noise
When prepared correctly, KBr pellets can produce exceptionally high-quality spectra with high signal-to-noise ratios. The resulting "classic" transmission spectrum is often considered the gold standard for creating spectral libraries.
ATR spectra are generally of very high quality as well, but the signal strength is dependent on the quality of contact between the sample and the crystal.
Pathlength and Peak Correction
In the KBr method, the pathlength is fixed by the thickness of the pellet. This results in peak intensities that are directly proportional to concentration.
In ATR, the effective pathlength is wavelength-dependent. The evanescent wave penetrates deeper into the sample at longer wavelengths (lower wavenumbers). This skews the spectrum, making peaks at lower wavenumbers appear artificially intense compared to a true transmission spectrum. Modern FTIR software includes a simple mathematical "ATR correction" to account for this effect.
Understanding the Trade-offs
Neither method is universally superior; the best choice depends entirely on your analytical goal and the nature of your sample.
KBr: The Challenge of Consistency
The primary drawback of the KBr method is its reliance on operator skill. Poor grinding can cause scattering of the IR beam, and moisture absorbed from the air will introduce large, broad water peaks that can obscure the sample spectrum. Achieving reproducible results requires consistent and careful preparation.
ATR: The Surface-Only Limitation
The greatest strength of ATR is also its main limitation: it is a surface analysis technique. The spectrum you obtain represents only the top few microns of your material. If the surface is coated, contaminated, or chemically different from the bulk material, ATR will not give you a representative analysis of the whole sample.
Making the Right Choice for Your Analysis
Use your primary objective to guide your decision between these powerful techniques.
- If your primary focus is high-quality library matching or quantitative analysis: The KBr method is often preferred for its classic transmission spectra and direct control over pathlength and concentration.
- If your primary focus is rapid screening or routine quality control: ATR is the undisputed winner due to its incredible speed, ease of use, and minimal sample preparation requirements.
- If you are analyzing difficult samples like liquids, pastes, or intractable polymers: ATR is far more versatile and allows for direct analysis without dilution or complex preparation steps.
Understanding this fundamental trade-off between meticulous preparation for quantitative depth and rapid analysis for surface characterization is the key to leveraging FTIR effectively in your work.
Summary Table:
| Feature | KBr (Transmission) Method | ATR (Attenuated Total Reflectance) Method |
|---|---|---|
| Principle | IR light passes through a prepared sample pellet. | IR light reflects off a crystal, analyzing the sample surface. |
| Sample Prep | Labor-intensive: grinding, pressing, moisture-sensitive. | Minimal: place sample on crystal and measure. |
| Analysis Depth | Bulk material analysis. | Surface-only (top 0.5-2 microns). |
| Best For | Quantitative analysis, library matching, high-quality spectra. | Rapid screening, quality control, liquids/pastes, routine analysis. |
Struggling to choose the right FTIR method for your specific materials? The experts at KINTEK can help you optimize your spectroscopy workflow. We specialize in providing the right lab equipment and consumables—from ATR accessories to pellet presses—to ensure accurate and efficient analysis for your laboratory.
Let us help you achieve superior results. Contact our team today to discuss your application and find the perfect solution.
Visual Guide
Related Products
- Infrared Heating Quantitative Flat Plate Press Mold
- Warm Isostatic Press for Solid State Battery Research
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Optical Window Glass Substrate Wafer Barium Fluoride BaF2 Substrate Window
- No Demolding Lab Infrared Press Mold for Laboratory Applications
People Also Ask
- What is the significance of using a laboratory hydraulic press in the assembly of all-solid-state pellet stack batteries?
- What is the function of a hydraulic forging press? Shape Metal with Unmatched Force and Control
- What is the purpose of a laboratory hydraulic press in FTIR analysis? Create High-Quality Transparent Pellets
- Why is a hydraulic press used to apply 380 MPa to battery bilayers? Achieve Superior Density & Safety
- What are the components of a plate and frame filter press? A Detailed Breakdown of the 4 Key Systems
- Is pressure constant in a hydraulic press? Unlock the Power of Force Multiplication
- What is the difference between a hydraulic press and an air press? Choose the Right Force for Your Application
- What is the purpose of using a laboratory hydraulic press? Achieve Precision in Diamond-Aluminum Powder Processing



















