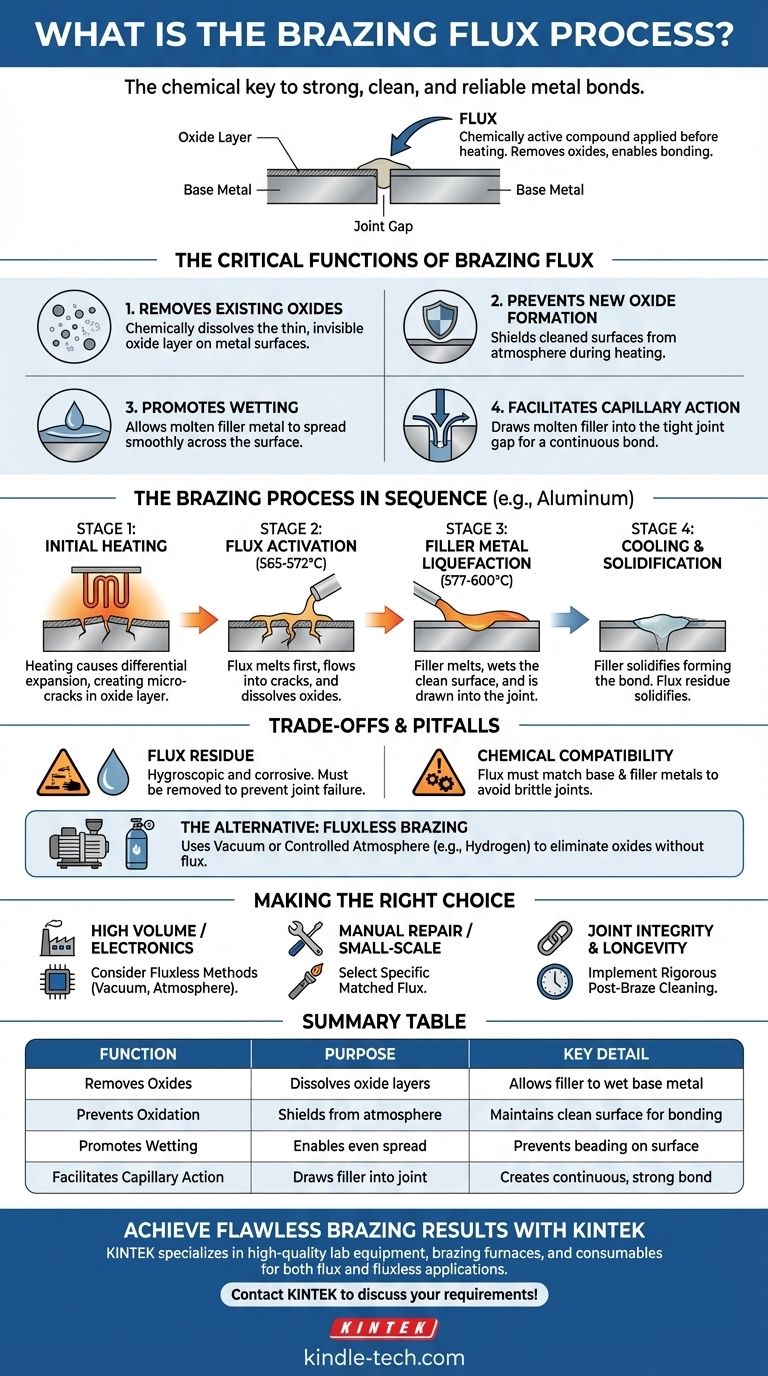
In any brazing operation, flux is a chemically active compound applied to the joint before heating. It melts at a lower temperature than the brazing filler metal, acting as a powerful cleaning agent that removes stubborn surface oxides. This chemical cleaning action is essential for allowing the molten filler metal to wet the base metals, flow into the joint via capillary action, and form a strong, continuous bond.
The core purpose of brazing flux is not merely to clean the joint, but to solve a fundamental chemistry problem: metals will not bond in the presence of an oxide layer. Whether you use a chemical flux or a controlled atmosphere, removing and preventing these oxides is the non-negotiable first step to creating a successful brazed joint.

The Critical Functions of Brazing Flux
To understand the brazing process, you must first understand the four distinct roles that flux plays. It is not a passive material but an active participant in the metallurgical process.
1. It Removes Existing Oxides
Nearly all metals, especially when heated, are covered by a thin, invisible layer of oxide. This layer acts as a barrier, preventing the molten filler metal from making direct contact with the pure base metal beneath. The flux's primary job is to chemically dissolve this oxide layer.
2. It Prevents New Oxide Formation
As you heat the parts to brazing temperature, the rate of oxidation increases dramatically. A layer of molten flux shields the cleaned metal surfaces from the surrounding atmosphere, preventing new, damaging oxides from forming during the heating cycle.
3. It Promotes Wetting
"Wetting" is the ability of a liquid to spread smoothly across a solid surface. Molten filler metal will bead up on an oxidized surface, much like water on a waxed car. By providing a chemically clean surface, flux allows the filler metal to "wet" the base metals and spread evenly.
4. It Facilitates Capillary Action
Once wetting is achieved, the molten filler metal can be drawn into the tight gap of the joint through a force known as capillary action. This force only works on a clean, wetted surface, making flux the enabler of this critical filling process.
The Brazing Process in Sequence
Using the common example of aluminum brazing, we can see how these principles play out in a timed, temperature-dependent sequence.
Stage 1: Initial Heating
As the assembly is heated, the base metal and the solid oxide layer on its surface expand at different rates. This differential expansion causes the brittle oxide layer to develop micro-cracks.
Stage 2: Flux Activation
At a specific temperature range, typically 565-572°C (1049-1062°F) for aluminum, the flux melts. It is engineered to become liquid before the filler metal does. The molten flux immediately flows into the micro-cracks, beginning its work of dissolving the oxide layer.
Stage 3: Filler Metal Liquefaction
As the temperature rises further to 577-600°C (1071-1112°F), the filler metal melts. It flows onto a surface that has already been cleaned and protected by the molten flux, allowing it to wet the base metal and be drawn fully into the joint.
Stage 4: Cooling and Solidification
Upon cooling, the filler metal solidifies, forming the permanent metallurgical bond. The flux also solidifies into a hard, glassy residue on and around the joint.
Understanding the Trade-offs and Pitfalls
While essential for many applications, using flux is not without its challenges. An expert understands both its benefits and its liabilities.
The Problem of Flux Residue
After brazing, the solidified flux residue is not benign. It is often hygroscopic (attracts moisture) and corrosive, which can lead to joint failure over time. This residue must be thoroughly removed through mechanical or chemical cleaning. Furthermore, it can hide joint defects from inspection and interfere with subsequent painting or plating.
The Importance of Chemical Compatibility
Flux is not a one-size-fits-all product. The flux chemistry must be compatible with both the base metal and the filler metal. Using a phosphorus-bearing filler alloy on an iron or nickel-based component, for example, can create brittle phosphides in the joint, severely compromising its strength. The flux must be selected for the specific materials being joined.
The Alternative: Fluxless Brazing
In many industrial settings, flux is eliminated entirely. Processes like vacuum brazing or controlled atmosphere brazing use the environment itself to solve the oxide problem. A vacuum removes the oxygen, while a specific gas atmosphere (like hydrogen) can chemically reduce oxides, achieving a clean surface without the need for flux and its associated cleanup.
Making the Right Choice for Your Application
Your approach should be dictated by your project's specific requirements for cleanliness, volume, and material compatibility.
- If your primary focus is high-volume production or sensitive electronics: Consider fluxless methods like vacuum or controlled atmosphere brazing to eliminate post-braze cleaning and ensure maximum joint cleanliness.
- If your primary focus is manual repair or small-scale fabrication: Select a flux that is specifically matched to your base metal, filler alloy, and heating method (e.g., torch vs. furnace).
- If your primary focus is joint integrity and longevity: You must implement a rigorous post-brazing cleaning process to remove all potentially corrosive flux residue after the operation is complete.
Ultimately, controlling the surface chemistry of the joint is the key to a successful braze, and flux is the most common chemical tool for achieving that control.
Summary Table:
| Function | Purpose | Key Detail |
|---|---|---|
| Removes Oxides | Dissolves surface oxide layers | Allows filler metal to wet the base metal |
| Prevents Oxidation | Shields metal from atmosphere during heating | Maintains a clean surface for bonding |
| Promotes Wetting | Enables filler metal to spread evenly | Prevents beading on the surface |
| Facilitates Capillary Action | Draws molten filler into the joint gap | Creates a continuous, strong bond |
Achieve flawless brazing results with the right equipment and expertise.
Brazing is a precise process where the right tools and consumables make all the difference. KINTEK specializes in high-quality lab equipment and consumables, including brazing furnaces and compatible materials, to serve your specific laboratory and fabrication needs.
Let our experts help you select the perfect solution for your application, whether you require flux-based systems or advanced fluxless alternatives like vacuum brazing. We are committed to helping you create strong, clean, and reliable joints with maximum efficiency.
Contact KINTEK today to discuss your brazing requirements and discover how we can enhance your process!
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- High Shear Homogenizer for Pharmaceutical and Cosmetic Applications
- Custom PTFE Teflon Parts Manufacturer for Reagent Wide Mouth Fine Mouth Sample High Temperature Bottles
- Custom PTFE Teflon Parts Manufacturer for PTFE Ball Valve Seat
- Custom PTFE Teflon Parts Manufacturer for Hollow Cleaning Basket and Rack Carrier
People Also Ask
- What is the difference between wet and dry sieve analysis? Choose the Right Method for Accurate Particle Sizing
- What are alloys in simple words? Unlock the Power of Engineered Materials
- What are the advantages and disadvantages of sieve analysis test? A Guide to Effective Particle Sizing
- What are the four main types of sensors? A Guide to Power Source and Signal Type
- What is the impact factor of powder metallurgy progress? A 2022 Analysis & Context



















