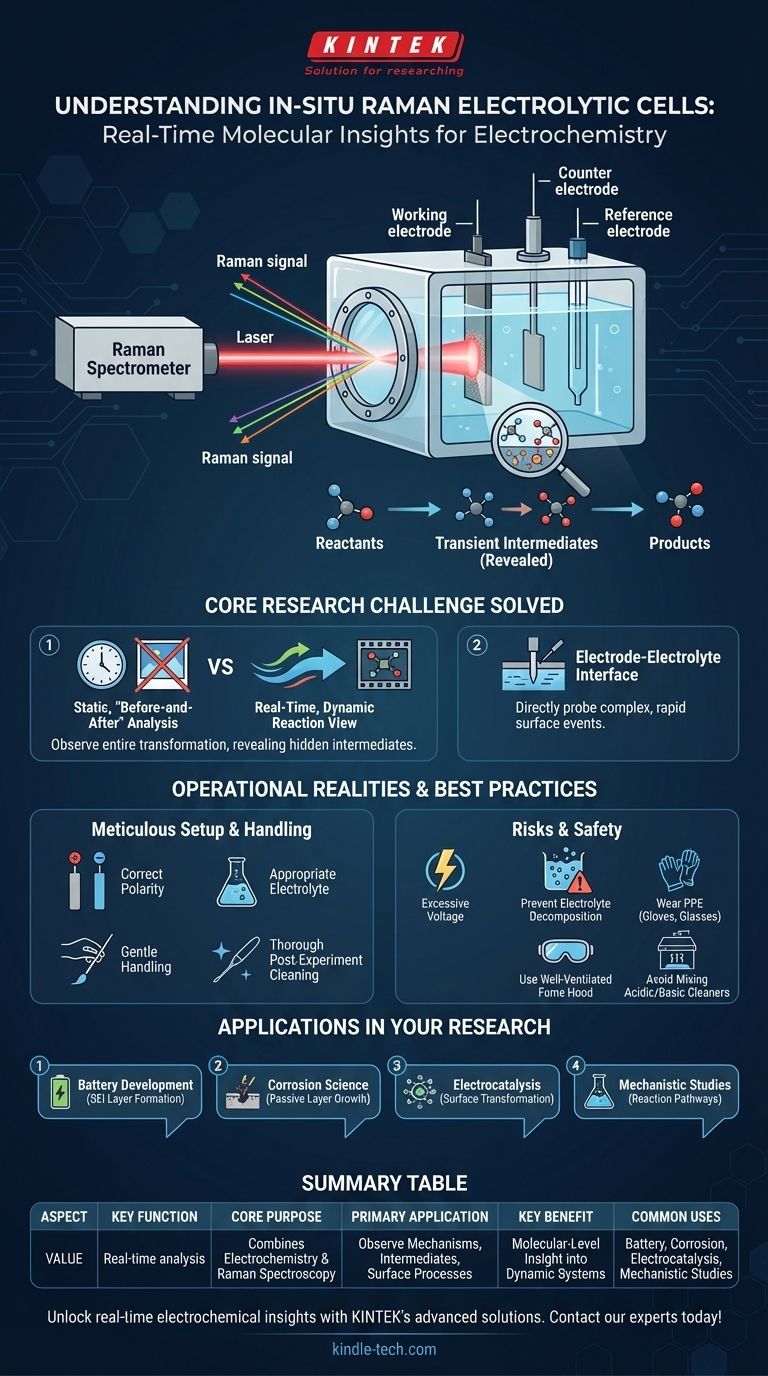
At its core, an in-situ Raman electrolytic cell is a specialized device that allows scientists to perform Raman spectroscopy directly on an electrochemical system while a reaction is in progress. It effectively combines an electrochemical workstation with a Raman spectrometer, providing a real-time, molecular-level view of processes occurring at the electrode surface and within the surrounding electrolyte.
This tool closes the gap between static, "before-and-after" analysis and the dynamic reality of a reaction. Instead of only knowing the starting and ending points, it allows you to watch the entire chemical transformation unfold, revealing transient intermediates and reaction mechanisms that would otherwise be invisible.

How It Solves a Core Research Challenge
The primary challenge in electrochemistry is understanding the complex and rapid events that happen at the electrode-electrolyte interface. The in-situ Raman cell is engineered specifically to overcome this obstacle.
Combining Electrochemistry and Spectroscopy
The device is fundamentally an electrochemical cell (with a working electrode, counter electrode, and reference electrode) built into a housing that allows a laser to be focused on the working electrode's surface. While the cell drives a reaction (like charging a battery or corroding a metal), the Raman spectrometer collects data from that exact spot.
Capturing Real-Time Data
The term "in-situ" means "in the original place" or "in position." This is the key advantage. You are not stopping the reaction to take a sample. For example, during metal electrodeposition, you can directly observe the depletion of metal ions in the electrolyte and the simultaneous formation of the new metallic layer on the electrode.
Probing the Electrode-Electrolyte Interface
This microscopic region is where all the critical action happens. The cell's design, often featuring a thin, transparent window, allows the Raman laser to precisely probe this boundary layer. This provides high-fidelity information about the chemical bonds of molecules right on the surface, revealing how they attach, change, and detach.
Understanding the Operational Realities
While powerful, the precision of an in-situ cell demands meticulous handling and an awareness of its limitations. Mistakes can compromise data integrity and even damage the equipment.
The Need for Meticulous Setup
The quality of your results is directly tied to the quality of your setup. You must ensure the correct electrode polarity is established to drive the intended reaction. Choosing an inappropriate electrolyte can introduce unwanted side reactions that obscure the process you want to study.
Risk of Electrode and Electrolyte Damage
Applying an excessively high voltage is a common pitfall. It can cause the electrolyte to decompose, generating bubbles or side products that interfere with the Raman signal. It can also cause irreversible physical or chemical damage to the electrode surface itself.
The Importance of Post-Experiment Care
Residue from a previous experiment is a source of contamination for the next one. Immediate and thorough cleaning after each use is non-negotiable to ensure data reproducibility. The cell's complex construction also means it must be handled gently to avoid misalignment or breakage.
Best Practices for Safe and Effective Operation
Proper protocol is essential for both user safety and the longevity of the cell.
Handling and Cleaning Protocols
During cleaning, never use metal brushes or other hard tools that could scratch the delicate electrode surface or the optical window, as scratches will scatter the laser light and ruin your signal. For long-term storage, ensure all components are clean and dry to prevent corrosion.
Chemical Safety is Paramount
Always wear appropriate personal protective equipment (PPE), such as gloves and safety glasses, when working with electrolytes, many of which are corrosive. All work should be performed in a well-ventilated fume hood. Critically, never mix acidic and basic cleaning agents, as this can cause a dangerous and violent exothermic reaction.
How to Apply This to Your Research
Your specific application will determine how you leverage the cell's capabilities.
- If your primary focus is mechanistic studies: Use the cell to identify short-lived intermediate species and map out the step-by-step pathway of a complex electrochemical reaction.
- If your primary focus is battery development: The cell is invaluable for observing the formation and degradation of the solid-electrolyte interphase (SEI) layer during charge-discharge cycles.
- If your primary focus is corrosion science: It allows you to watch the initial stages of metal oxidation and the formation of passive, protective layers in real time.
- If your primary focus is electrocatalysis: You can directly observe how reactant molecules adsorb onto a catalyst's surface and transform into products.
Ultimately, this powerful tool transforms the electrode-electrolyte interface from a theoretical concept into a directly observable scientific environment.
Summary Table:
| Aspect | Key Function |
|---|---|
| Core Purpose | Combines electrochemistry with Raman spectroscopy for real-time analysis. |
| Primary Application | Observes reaction mechanisms, intermediates, and surface processes. |
| Key Benefit | Provides molecular-level insight into dynamic electrochemical systems. |
| Common Uses | Battery development, corrosion science, electrocatalysis, and mechanistic studies. |
Ready to unlock real-time insights into your electrochemical research? KINTEK specializes in high-quality lab equipment and consumables, including advanced electrochemical cells tailored for in-situ analysis. Our solutions are designed to help researchers like you achieve precise, reliable data. Contact our experts today to find the perfect equipment for your laboratory needs!
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- H Type Electrolytic Cell Triple Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Thin-Layer Spectral Electrolysis Electrochemical Cell
People Also Ask
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control
- What is a H type cell? A Guide to Divided Electrochemical Cells for Accurate Experiments



















