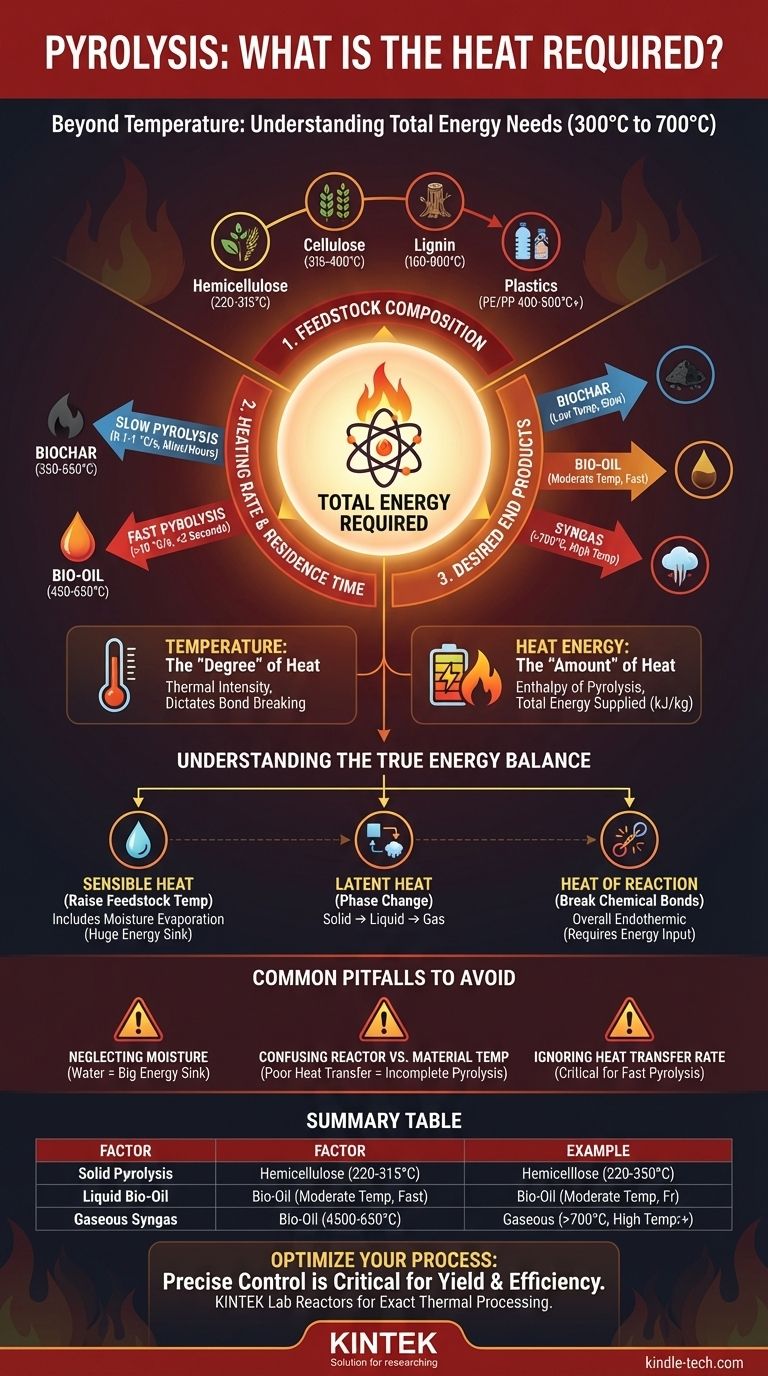
To be precise, pyrolysis is not defined by a single temperature but occurs over a range, typically between 300°C and 700°C (572°F to 1292°F) in an oxygen-free environment. The specific heat required depends entirely on the feedstock material, the desired end products, and the speed of the process. Simply aiming for a temperature misses the more critical metric: the total energy needed to drive the chemical decomposition.
The core issue is shifting from "What temperature do I need?" to "What is the total energy required for my specific goal?" This energy balance is governed by feedstock properties, heating rate, and your target products—biochar, bio-oil, or syngas.

Differentiating Temperature from Heat Energy
A common point of confusion is equating the process temperature with the total heat required. These are related but distinct concepts that are critical to understand for designing or operating any pyrolysis system.
Temperature: The "Degree" of Heat
Temperature is a measure of the thermal intensity within the reactor. It dictates which chemical bonds can be broken and influences the rate of reaction. Different temperatures favor the formation of different products.
Heat Energy: The "Amount" of Heat
Heat energy, or the enthalpy of pyrolysis, is the total amount of energy (often measured in kJ/kg) that must be supplied to the feedstock to raise its temperature and drive the chemical reactions. This is the true "heat required" and is what determines your energy costs and reactor design.
Key Factors Influencing the Heat Requirement
The "right" temperature and energy input are not fixed values. They are variables you control to achieve a specific outcome.
Feedstock Composition
Different materials break down at different temperatures. For biomass, the primary components decompose across distinct ranges:
- Hemicellulose: 220-315°C
- Cellulose: 315-400°C
- Lignin: 160-900°C (decomposes slowly across a very wide range)
Plastics also vary significantly. Polyethylene (PE) and Polypropylene (PP) require temperatures around 400-500°C, while a more stable polymer like PET requires higher temperatures.
Heating Rate and Residence Time
The speed at which you heat the material is one of the most important process parameters.
- Slow Pyrolysis: Uses low heating rates (0.1-1 °C/s) and long residence times (minutes to hours). This process operates at lower temperatures (350-550°C) and maximizes the yield of biochar.
- Fast Pyrolysis: Uses extremely high heating rates (>10 °C/s) and very short residence times (<2 seconds). This requires higher temperatures (450-650°C) to rapidly break down material and is optimized for producing liquid bio-oil.
Desired End Products
Your target output dictates the process conditions.
- For Biochar: Lower temperatures and slow heating preserve the fixed carbon structure.
- For Bio-oil: Higher temperatures and rapid heating break down the feedstock into vapors, which are then quickly cooled and condensed into liquid.
- For Syngas: Very high temperatures (>700°C) are needed to "crack" the larger molecules (including pyrolysis vapors) into smaller, non-condensable gas molecules like hydrogen and carbon monoxide.
Understanding the True Energy Balance
The total heat you must supply can be broken down into three distinct needs.
1. Sensible Heat for Heating
This is the energy needed to raise the feedstock from its starting temperature to the target pyrolysis temperature. A significant portion of this is often used just to boil off any moisture, which requires a large amount of energy.
2. Latent Heat for Phase Change
This is the energy required to convert solids into liquids and liquids into gases. For dry feedstock, this is primarily the energy needed to vaporize the decomposing material.
3. Heat of Reaction
Pyrolysis is, overall, an endothermic process, meaning it requires a net input of energy to break the strong chemical bonds in the feedstock. While some secondary reactions that form new molecules can be exothermic (releasing heat), the overall process balance always requires energy input.
Common Pitfalls to Avoid
Achieving the correct thermal conditions is more complex than just setting a thermostat.
Neglecting Feedstock Moisture
Water is an enormous energy sink. A feedstock with 20% moisture will require substantially more energy input than one with 5% moisture, as all that water must be evaporated before the material can reach pyrolysis temperatures.
Confusing Reactor vs. Material Temperature
The temperature of your reactor wall is not the temperature inside a wood chip or piece of plastic. Poor heat transfer can mean the core of your feedstock is much cooler than the reactor setpoint, leading to incomplete pyrolysis and undesirable products.
Ignoring Heat Transfer Rate
For fast pyrolysis, the rate at which you can transfer heat into the feedstock particle is paramount. If you cannot supply energy quickly enough, you will inadvertently be performing slow pyrolysis, regardless of your reactor's temperature setting.
Making the Right Choice for Your Goal
Instead of asking for a single temperature, define your objective first. The optimal conditions will follow from your goal.
- If your primary focus is maximizing biochar yield: Use lower temperatures (350-550°C) and a slow heating rate to preserve the carbon structure.
- If your primary focus is maximizing bio-oil production: Use moderate-to-high temperatures (450-650°C) with a very high heating rate and short vapor residence time.
- If your primary focus is maximizing syngas production: Use high temperatures (>700°C) to ensure the complete thermal cracking of all vapors into simple gas molecules.
Ultimately, mastering pyrolysis is about precisely controlling the flow of energy to guide the material toward your desired chemical outcome.
Summary Table:
| Factor | Impact on Heat Requirement | Typical Range/Example |
|---|---|---|
| Feedstock Type | Different materials decompose at different temperatures and energies. | Biomass: 300-700°C; Plastics: 400-500°C+ |
| Target Product | Dictates the optimal temperature and heating rate. | Biochar (low temp, slow); Bio-oil (moderate temp, fast) |
| Heating Rate | Faster rates require higher power input for the same mass. | Slow: 0.1-1 °C/s; Fast: >10 °C/s |
| Moisture Content | High moisture significantly increases energy needed for evaporation. | 20% moisture vs. 5% moisture |
Ready to optimize your pyrolysis process? The precise control of temperature and heat transfer is critical for yield and efficiency. KINTEK specializes in high-quality lab reactors and furnaces designed for exact thermal processing. Whether you're researching biochar, bio-oil, or syngas production, our equipment ensures reliable, repeatable results. Contact our experts today to discuss your specific application and find the perfect solution for your laboratory's needs.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Laboratory High Pressure Vacuum Tube Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- What is the temperature range for calcination? Master the 800°C to 1300°C Process
- What machine is used for calcination? The Essential Role of the Calciner in Industrial Processing
- What are the negative effects of pyrolysis? High Costs and Environmental Risks Explained
- What plastics can be used in pyrolysis? A Guide to Ideal Feedstocks for Waste-to-Energy
- What is the composition of fast pyrolysis oil? A Guide to the Complex Chemical Intermediate
- What are the components of a rotary kiln? A Guide to the Core Systems and Parts
- What are the different types of pyrolysis reactors? Choose the Right Reactor for Your Process
- What are the three types of pyrolysis process? Slow, Fast, and Conventional Explained



















