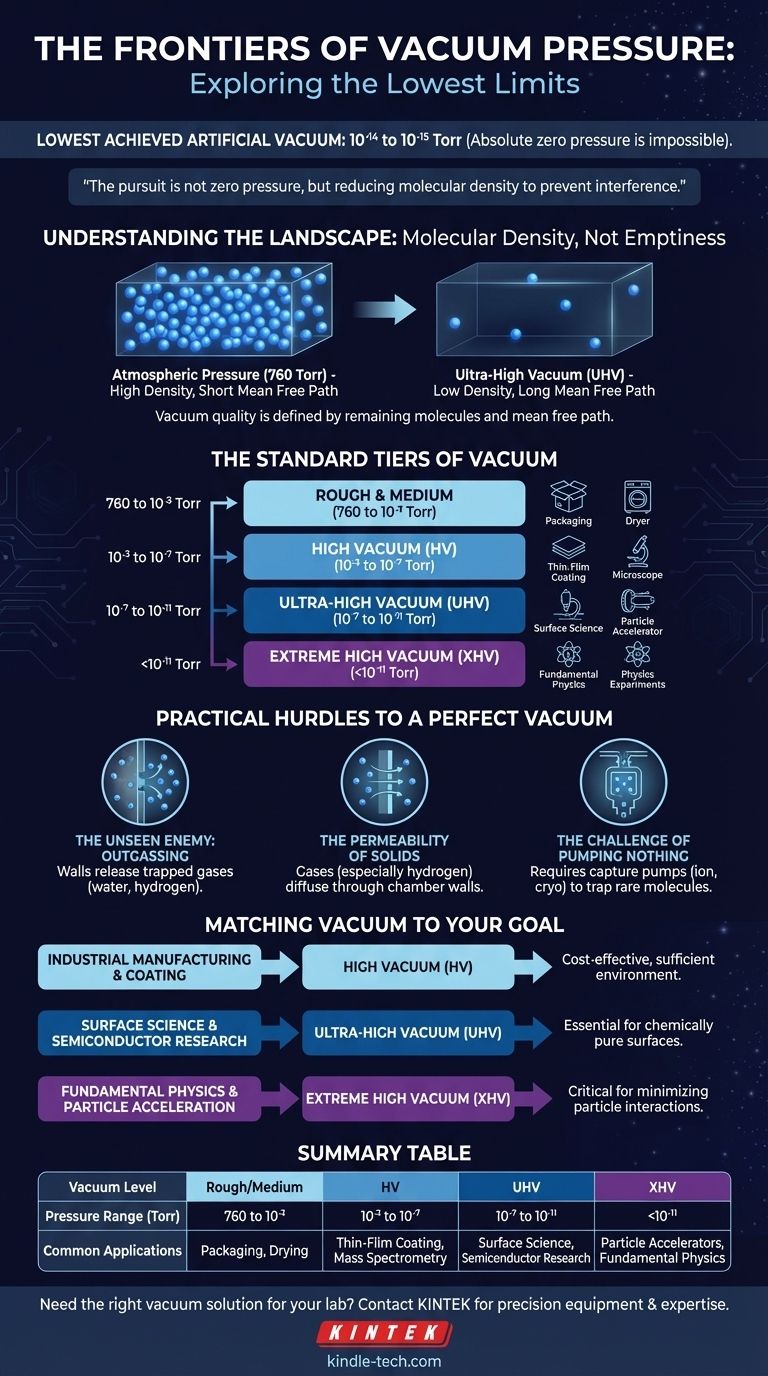
The lowest possible vacuum pressure achieved in an artificial setting is in the range of 10⁻¹⁴ to 10⁻¹⁵ Torr. While specialized laboratories can routinely reach pressures of 10⁻¹² to 10⁻¹³ Torr, achieving an absolute vacuum—a pressure of zero—is considered physically impossible.
The pursuit of a perfect vacuum is not about reaching zero pressure. Instead, it's a technical endeavor to reduce the density of gas molecules to a level where they no longer interfere with a specific scientific or industrial process.

Understanding the Landscape of a Vacuum
It's About Molecular Density, Not Emptiness
A "vacuum" isn't a state of complete nothingness. It's a space containing gas molecules at a pressure significantly lower than the surrounding atmosphere. Standard atmospheric pressure is approximately 760 Torr (or 1000 mbar).
The quality of a vacuum is defined by how few molecules remain. As you move to lower pressures, the distance a single molecule can travel before hitting another—its mean free path—increases dramatically.
The Standard Tiers of Vacuum
Vacuum levels are categorized based on the pressure range, with each tier enabling progressively more sensitive applications.
- Rough & Medium Vacuum (760 to 10⁻³ Torr): This range is used for mechanical tasks like food packaging, drying, and distillation. The number of molecules is reduced, but they are still very dense.
- High Vacuum (HV) (10⁻³ to 10⁻⁷ Torr): At this level, the mean free path of molecules becomes significant. This is critical for processes like thin-film coating, mass spectrometry, and operating electron microscopes.
- Ultra-High Vacuum (UHV) (10⁻⁷ to 10⁻¹¹ Torr): In UHV, molecules are so sparse that a particle can travel kilometers before a collision. This pristine environment is essential for surface science, particle accelerators, and fundamental physics research.
- Extreme High Vacuum (XHV) (<10⁻¹¹ Torr): This is the frontier of vacuum technology. Reaching XHV requires specialized equipment and techniques to battle the physical limits of materials themselves.
The Practical Hurdles to a Perfect Vacuum
Achieving progressively lower pressures becomes exponentially more difficult. The primary challenge shifts from simply removing air to fighting against the physics of the container itself.
The Unseen Enemy: Outgassing
The biggest barrier to achieving UHV and XHV is outgassing. The walls of the vacuum chamber, even if made of highly polished stainless steel, contain trapped gases like water vapor and hydrogen. These molecules are slowly released from the material's surface, constantly adding gas back into the system.
The Permeability of Solids
At extreme low pressures, gases from the outside atmosphere can diffuse or permeate directly through the solid walls of the vacuum chamber. Hydrogen, being the smallest molecule, is particularly problematic and can slowly seep through even dense metal.
The Challenge of Pumping Nothing
Conventional pumps work by moving a fluid, but at UHV levels, there is no continuous fluid of gas. The system must instead capture individual, randomly moving molecules. This requires specialized capture pumps, such as ion pumps or cryopumps, which trap molecules rather than expelling them.
Matching the Vacuum to Your Goal
The "best" vacuum is the one that meets the requirements of your application without excessive cost and complexity.
- If your primary focus is industrial manufacturing or coating: A High Vacuum (HV) provides the necessary environment for most processes without the extreme costs of UHV systems.
- If your primary focus is surface science or semiconductor research: An Ultra-High Vacuum (UHV) is non-negotiable to maintain a chemically pure surface for analysis or deposition.
- If your primary focus is fundamental physics or particle acceleration: Pushing into Extreme High Vacuum (XHV) is essential to minimize unwanted particle interactions and ensure experimental accuracy.
Ultimately, selecting the right vacuum level is a critical engineering decision that balances technical requirements against the fundamental physical limitations of matter.
Summary Table:
| Vacuum Level | Pressure Range (Torr) | Common Applications |
|---|---|---|
| Rough/Medium | 760 to 10⁻³ | Packaging, Drying |
| High Vacuum (HV) | 10⁻³ to 10⁻⁷ | Thin-Film Coating, Mass Spectrometry |
| Ultra-High Vacuum (UHV) | 10⁻⁷ to 10⁻¹¹ | Surface Science, Semiconductor Research |
| Extreme High Vacuum (XHV) | <10⁻¹¹ | Particle Accelerators, Fundamental Physics |
Need the right vacuum solution for your lab? At KINTEK, we specialize in precision lab equipment and consumables tailored to your unique requirements. Whether you're working in industrial manufacturing, semiconductor research, or fundamental physics, our expertise ensures you achieve the optimal vacuum environment for your process. Contact us today to discuss how we can support your laboratory's success with reliable, high-performance vacuum systems.
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
- Electric Heated Hydraulic Vacuum Heat Press for Lab
- Variable Speed Peristaltic Pump
- Laboratory Rotary Vane Vacuum Pump for Lab Use
People Also Ask
- What are the advantages of a water circulating vacuum pump? Superior Durability for Demanding Lab Environments
- Why is a water circulating vacuum pump suitable for handling flammable or explosive gases? Inherent Safety Through Isothermal Compression
- What can I use a vacuum pump for? Powering Industrial Processes from Packaging to Automation
- How is a circulating water vacuum pump utilized for hydrogen production residues? Optimize Your Solid-Liquid Separation
- How does a water circulating vacuum pump operate? Discover the Efficient Liquid Piston Principle



















