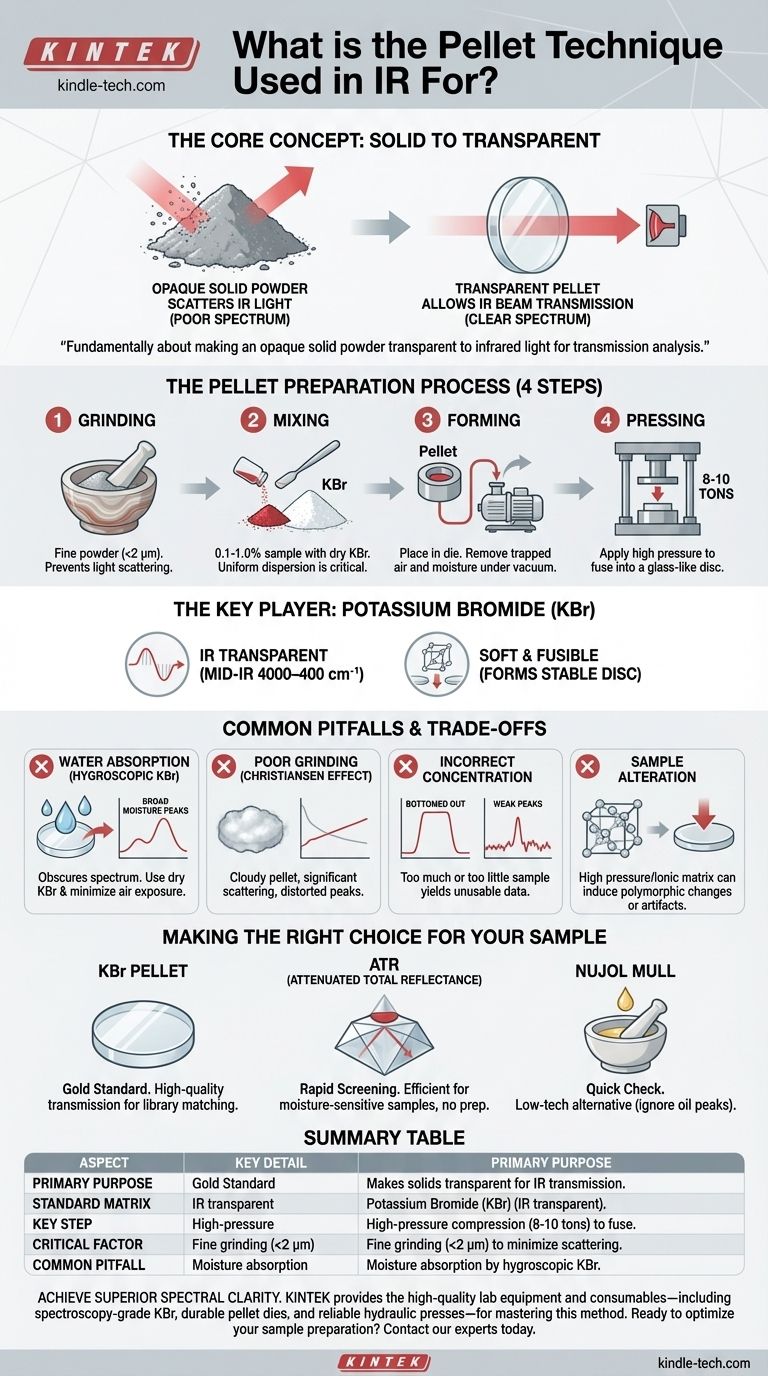
In infrared (IR) spectroscopy, the pellet technique is a well-established method for preparing solid samples for analysis. Its purpose is to transform a solid, which is typically opaque and scatters light, into a thin, semi-transparent disc that allows the IR beam to pass through it for a transmission measurement. This disc is created by mixing a small amount of the sample with a dry, IR-transparent salt, most commonly potassium bromide (KBr), and compressing the mixture under high pressure.
The KBr pellet technique is fundamentally about solving a physical problem: making an opaque solid powder transparent to infrared light. It achieves this by dispersing the analyte at a low concentration within a salt matrix that, under pressure, fuses into a glass-like disc suitable for transmission analysis.
The Challenge of Solid Sample Analysis
The Problem with Powders
The most common form of IR spectroscopy, transmission, requires the infrared beam to pass directly through the material being analyzed. Solid samples, especially fine powders, scatter the majority of the IR light, preventing it from reaching the detector and yielding a poor or unusable spectrum.
The Goal: Optical Clarity
The pellet method overcomes this by embedding the sample particles within a matrix that has a similar refractive index. When ground finely and subjected to immense pressure, the mixture fuses, minimizing scattering at the particle boundaries and allowing light to pass through.
The Role of Potassium Bromide (KBr)
Potassium bromide (KBr) is the standard choice for the matrix material for two key reasons. First, it is transparent to mid-range infrared radiation (roughly 4000 cm⁻¹ to 400 cm⁻¹), meaning it does not have its own absorption peaks that would interfere with the sample's spectrum. Second, it is a relatively soft crystalline solid that flows and fuses under pressure to form a stable, transparent pellet.
The Pellet Preparation Process
Step 1: Grinding
The sample and the KBr must both be ground into an extremely fine powder, ideally using an agate mortar and pestle. The goal is to reduce the particle size of the sample to less than the wavelength of the IR light being used (typically <2 µm) to prevent light scattering.
Step 2: Mixing
A very small amount of the ground sample (typically 0.1% to 1.0% by weight) is thoroughly mixed with a larger amount of dry, spectroscopy-grade KBr powder. Uniform dispersion is critical for a high-quality spectrum.
Step 3: Forming the Pellet
The mixture is placed into a specialized pellet die. The die is often attached to a vacuum line to remove trapped air and, more importantly, atmospheric moisture, which can obscure the spectrum.
Step 4: Applying Pressure
A hydraulic press is used to apply several tons of force (e.g., 8-10 tons) to the die. This immense pressure causes the KBr mixture to fuse into a solid, glass-like disc that is transparent or translucent. This finished disc can then be placed in a sample holder and analyzed in the spectrometer.
Understanding the Trade-offs and Common Pitfalls
The Primary Enemy: Water
KBr is hygroscopic, meaning it readily absorbs moisture from the air. Water has very strong and broad IR absorption bands (a wide peak around 3400 cm⁻¹ and another near 1640 cm⁻¹) that can easily overwhelm the peaks from your sample. Using dry KBr and minimizing exposure to air is essential.
Poor Grinding and Scattering Effects
If the sample is not ground finely enough, the resulting pellet will appear cloudy. This leads to significant light scattering, which manifests in the spectrum as a sloping baseline that is high on the left (high wavenumber) and low on the right (low wavenumber). This phenomenon, known as the Christiansen effect, distorts peak shapes and intensities.
Incorrect Sample Concentration
Using too much sample will result in absorption peaks that are too intense ("bottomed out"), where nearly all the light at that frequency is absorbed. Conversely, using too little sample will produce a noisy spectrum with weak peaks that are difficult to distinguish from the baseline.
Potential for Sample Alteration
The extremely high pressures used to form the pellet can occasionally induce changes in the sample, such as altering its crystalline form (polymorphism). Furthermore, the ionic nature of the KBr matrix can interact with certain samples, leading to spectral artifacts or shifts in peak positions.
Making the Right Choice for Your Sample
When preparing a solid for IR analysis, the KBr pellet is a classic technique, but modern alternatives exist. Your choice depends on your goal and available equipment.
- If your primary focus is a high-quality, "textbook" transmission spectrum for library matching: The KBr pellet method, when executed carefully, remains the gold standard for producing clean, artifact-free data.
- If your primary focus is rapid screening or analyzing a moisture-sensitive sample: Attenuated Total Reflectance (ATR) is the most efficient choice, as it requires virtually no sample preparation beyond pressing the solid against the ATR crystal.
- If your primary focus is a quick check without a press or ATR: A Nujol mull (grinding the sample in mineral oil) is a viable low-tech alternative, but you must be prepared to identify and ignore the oil's own C-H absorption peaks in the spectrum.
Ultimately, understanding the principles behind each sample preparation method empowers you to translate a physical substance into clear, actionable spectral data.

Summary Table:
| Aspect | Key Detail |
|---|---|
| Primary Purpose | Makes solid samples transparent to IR light for transmission analysis. |
| Standard Matrix | Potassium Bromide (KBr), transparent in the mid-IR range. |
| Key Step | High-pressure compression (e.g., 8-10 tons) to fuse the mixture. |
| Critical Factor | Fine grinding (<2 µm) to minimize light scattering. |
| Common Pitfall | Moisture absorption by hygroscopic KBr, obscuring the spectrum. |
Achieve superior spectral clarity in your lab. The KBr pellet technique is fundamental for reliable IR analysis of solid samples. KINTEK specializes in providing the high-quality lab equipment and consumables—including spectroscopy-grade KBr, durable pellet dies, and reliable hydraulic presses—you need to master this method. Our expertise supports laboratories in obtaining accurate, reproducible results. Ready to optimize your sample preparation? Contact our experts today to discuss your specific requirements.
Visual Guide
Related Products
- kbr pellet press 2t
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Laboratory Manual Hydraulic Pellet Press for Lab Use
People Also Ask
- What is KBr disc method in IR spectroscopy? A Guide to Solid Sample Analysis
- How do you prepare a KBr pellet for IR spectroscopy? Master the Key Steps for a Clear Spectrum
- What is a KBr pellet? A Guide to Preparing Solid Samples for IR Spectroscopy
- What are the safety precautions for KBr? Achieve Flawless FTIR Pellet Preparation and Data Accuracy
- How much sample is needed for IR? Optimize Your Analysis with Minimal Material



















