In short, a polishing cloth for electrodes is a specialized, textured material used with an abrasive slurry to mechanically clean and smooth an electrode's surface. This process is not simple cleaning; it is a critical step in electrochemistry to remove microscopic contaminants and surface defects, ensuring the electrode provides an active, reproducible, and uniform surface for accurate measurements.
The core principle is that the reliability of any electrochemical measurement is fundamentally limited by the quality of the electrode surface. The polishing cloth, paired with the correct abrasive, is the primary tool used to prepare this surface to a pristine and repeatable state.

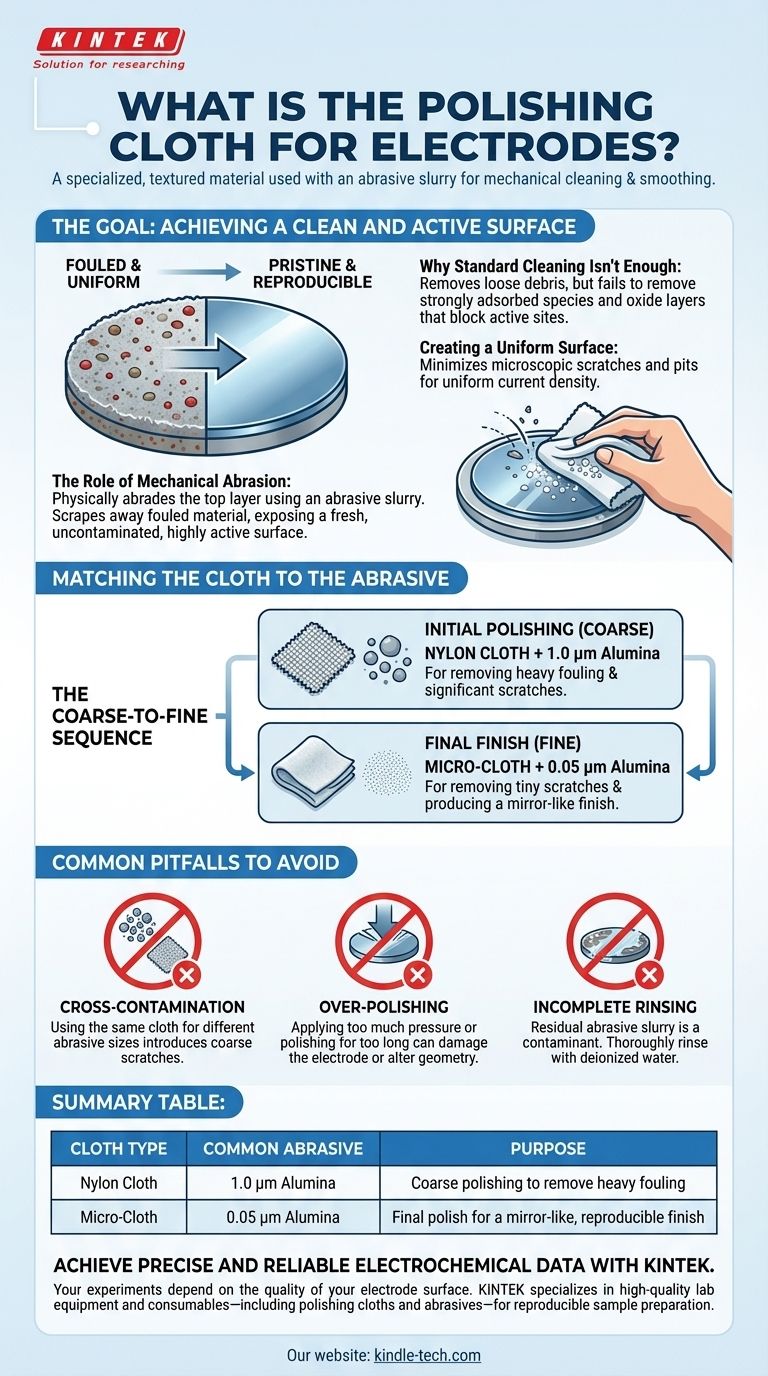
The Goal: Achieving a Clean and Active Surface
The entire purpose of polishing is to reset the electrode to a known, baseline condition before an experiment. An electrode's surface is where all the critical chemical reactions occur, and its condition can change dramatically during use.
Why Standard Cleaning Isn't Enough
Simple rinsing with a solvent might remove loose debris, but it fails to remove strongly adsorbed chemical species from previous experiments or the thin oxide layers that can form on the surface. These contaminants can block active sites, altering the electrode's electrochemical response.
The Role of Mechanical Abrasion
Polishing works by physically abrading the top microscopic layer of the electrode material. The polishing cloth holds an abrasive slurry (like alumina powder mixed with water) against the electrode. This action scrapes away fouled material, exposing a fresh, uncontaminated, and highly active surface underneath.
Creating a Uniform Surface
A secondary goal is to create a smooth, mirror-like finish. Microscopic scratches and pits can create areas of non-uniform current density, which can complicate the interpretation of results. A fine polish minimizes these physical defects.
Matching the Cloth to the Abrasive
The polishing process is typically a sequence, moving from a coarser abrasive to a finer one. The type of cloth is specifically designed to work with a certain size of abrasive particle.
The Coarse-to-Fine Sequence
Most polishing protocols start with a larger particle size to remove significant fouling or larger scratches, followed by progressively smaller particles to achieve a final, smooth finish. Using a fine abrasive from the start would be inefficient for a heavily contaminated electrode.
Nylon Cloth for Initial Polishing
A nylon polishing cloth, which is relatively hard and durable, is typically used for the initial, coarser polishing steps. It is paired with larger abrasive particles, such as 1.0 µm alumina powder, to effectively remove significant surface contamination.
Micro-Cloth for Final Finish
A micro-polishing cloth (often a felt-like material) is much softer and is used for the final, delicate polishing stages. This cloth is paired with very fine abrasives, like 0.3 µm or 0.05 µm alumina powder, to remove the tiny scratches left by the previous step and produce a mirror-like finish.
Common Pitfalls to Avoid
Proper polishing is a technique that requires care. Simply rubbing the electrode is not enough and can introduce new problems if done incorrectly.
Cross-Contamination
Never use the same cloth for different abrasive sizes. Using a cloth that previously held 1.0 µm particles for your final 0.05 µm polishing step will introduce coarse scratches and ruin the mirror finish.
Over-Polishing
Applying too much pressure or polishing for too long can damage the electrode. It can alter the electrode's geometry, embed abrasive particles into the surface, or, for softer materials like gold, excessively wear down the material.
Incomplete Rinsing
After polishing, the electrode must be thoroughly rinsed, typically with deionized water, and sometimes sonicated. Any residual abrasive slurry left on the surface is a contaminant that will interfere with your experiment.
Making the Right Choice for Your Protocol
Your specific polishing needs depend on the state of your electrode and the sensitivity of your experiment.
- If your primary focus is routine cleaning after a standard experiment: A quick final polish using a micro-cloth and 0.05 µm alumina is often sufficient to restore the surface.
- If your electrode is new or heavily fouled: Begin with a coarser step, using a nylon cloth with 1.0 µm alumina, before moving to a final polish with a separate micro-cloth and 0.05 µm alumina.
- If you are performing highly sensitive surface chemistry: Always finish with the finest available abrasive (e.g., 0.05 µm) on a dedicated, clean micro-cloth to achieve the most pristine and reproducible surface possible.
Mastering your polishing technique is the first and most critical step toward achieving high-quality, reliable electrochemical data.
Summary Table:
| Cloth Type | Common Abrasive | Purpose |
|---|---|---|
| Nylon Cloth | 1.0 µm Alumina | Coarse polishing to remove heavy fouling |
| Micro-Cloth | 0.05 µm Alumina | Final polish for a mirror-like, reproducible finish |
Achieve precise and reliable electrochemical data with KINTEK.
Your experiments depend on the quality of your electrode surface. KINTEK specializes in the high-quality lab equipment and consumables—including polishing cloths and abrasives—that are essential for reproducible sample preparation.
Let our experts help you select the right materials for your specific protocol. Contact our team today to discuss your laboratory needs and ensure your measurements start with a pristine surface.
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
People Also Ask
- What are the key characteristics of cathode materials for Electro-Fenton? Unlock High-Efficiency Radical Production
- What regular maintenance is required for an RVC sheet? A Guide to Preserving Electrode Performance
- Why are graphite brushes and carbon felt preferred as anode materials for MECs? Optimize Your Biofuel Performance
- What are the required operational steps before using a titanium electrode? Ensure Longevity and Efficiency
- What are the proper procedures for handling a titanium electrode after use? Ensure Longevity and Peak Performance
- Why is a stainless steel cathode utilized in electrochemical oxidation? Enhance Wastewater Treatment Efficiency
- What is glassy carbon used for? A Guide to Its Unique Properties and Key Applications
- What are the application areas for the Platinum-Titanium Functional Electrode? A Guide to High-Performance Electrochemical Solutions



















