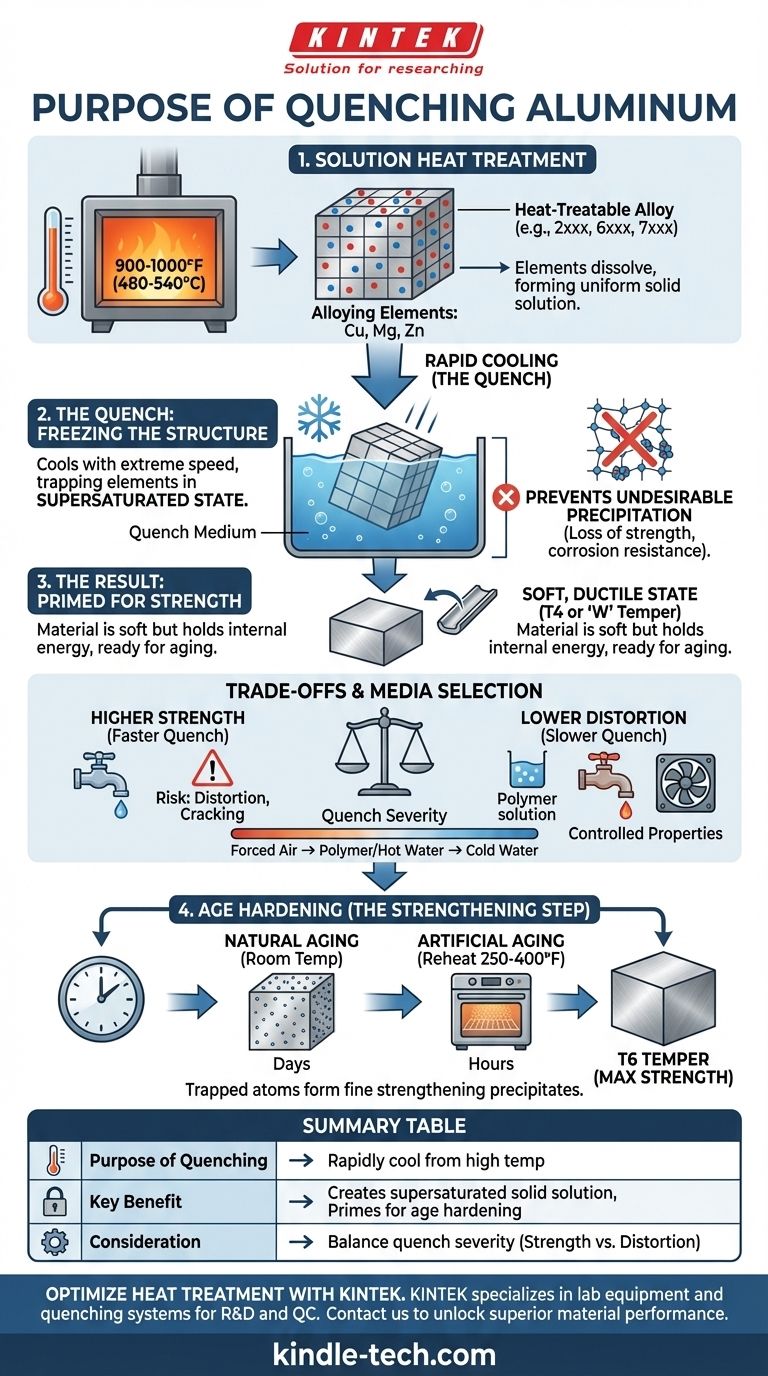
The fundamental purpose of quenching aluminum is to rapidly cool it from a high temperature to "freeze" its internal crystal structure in an unstable, supersaturated state. This process traps alloying elements like copper or silicon within the aluminum's atomic lattice, preventing them from precipitating out prematurely. This supersaturated condition is the critical prerequisite for a subsequent strengthening process known as age hardening.
Quenching does not make aluminum strong on its own. Instead, it is a preparatory step that traps the potential for strength within the metal, which is then unlocked through a later process called aging or precipitation hardening.

The Foundation: Solution Heat Treatment
To understand quenching, you must first understand the process it is part of: solution heat treatment. This process is only applicable to specific "heat-treatable" aluminum alloys, such as those in the 2xxx, 6xxx, and 7xxx series.
The Role of Alloying Elements
Heat-treatable alloys contain elements (like copper, magnesium, and zinc) that have limited solubility in aluminum at room temperature but can dissolve at elevated temperatures. Think of this like sugar in water—you can dissolve far more sugar in hot water than in cold water.
Step 1: The Solutionizing Heat
The first step is to heat the aluminum to a specific high temperature (typically around 900-1000°F or 480-540°C). This is held for a set time to allow the alloying elements to fully dissolve into the aluminum matrix, forming a uniform solid solution. At this point, the alloy's strengthening potential is fully "in solution."
The Critical Moment: The Purpose of the Quench
Once the alloying elements are dissolved, the material must be cooled with extreme speed. This rapid cooling is the quench.
Freezing the Supersaturated State
The quench cools the alloy so quickly that the dissolved atoms do not have time to cluster together and precipitate out of the solution. This traps them within the aluminum's crystal lattice, creating a supersaturated solid solution. This state is metallurgically unstable and holds a great deal of internal energy, much like a compressed spring.
Preventing Undesirable Precipitation
If the cooling is too slow, the alloying elements will begin to precipitate along the metal's grain boundaries. This form of precipitation is uncontrolled and detrimental, leading to a significant loss of strength and reduced corrosion resistance. The quench's speed is calculated to be faster than this critical cooling rate.
The Result: A Soft but Primed Material
Immediately after quenching, the aluminum is in its softest, most ductile state (known as the T4 or 'W' temper). While it's not strong, it is now perfectly primed for the final strengthening step.
Understanding the Trade-offs and Risks
The quenching process is a delicate balance. The rate of cooling is the most important variable and presents a classic engineering trade-off.
Quench Severity and Strength
A faster quench generally results in a better supersaturated solution, leading to higher potential strength after aging. Cold water provides a very severe quench and maximum strength potential.
The Risk of Distortion and Residual Stress
The primary downside of a very rapid quench is thermal shock. The extreme temperature gradient between the part's surface and core causes internal stresses that can lead to warping, distortion, and even cracking, especially in complex or thin-walled parts.
Choosing a Quench Medium
To manage this risk, different quench media are used:
- Cold Water: Highest cooling rate, highest risk of distortion.
- Hot Water: Less severe than cold water, reducing stress while still being effective for many alloys.
- Polymer Solutions: Provides a cooling rate between water and air, offering a good balance of strength and distortion control.
- Forced Air: A much slower quench used for very thin parts or alloys less sensitive to cooling rates.
The Final Step: Unlocking Strength Through Aging
The soft, quenched material achieves its final high-strength properties through a process called age hardening (or precipitation hardening).
Natural vs. Artificial Aging
Natural aging occurs when the quenched part is left at room temperature. Over several days, the trapped atoms will slowly begin to form tiny, highly dispersed strengthening precipitates on their own.
Artificial aging accelerates this process. The part is reheated to a low temperature (e.g., 250-400°F or 120-205°C) for several hours. This provides just enough thermal energy for the trapped atoms to move and form an optimal dispersion of microscopic precipitates that impede dislocation movement, dramatically increasing the alloy's strength and hardness. This is how common tempers like T6 are achieved.
Making the Right Choice for Your Goal
The choice of quenching method is dictated by the desired balance between mechanical properties and dimensional stability.
- If your primary focus is maximum strength and hardness: An aggressive quench in cold or cool water is necessary to achieve the best response to aging, but plan for potential post-quench straightening or stress relief.
- If your primary focus is minimizing distortion in a complex part: A less severe quench using a polymer solution, hot water, or even forced air may be required, accepting a predictable and controlled reduction in peak strength.
- If you are working with non-heat-treatable alloys (e.g., 3xxx or 5xxx series): Quenching serves no strengthening purpose, as these alloys gain their strength through work-hardening (strain), not heat treatment.
Ultimately, mastering the quench is essential for unlocking the full performance potential engineered into heat-treatable aluminum alloys.
Summary Table:
| Purpose of Quenching | Key Benefit | Consideration |
|---|---|---|
| Rapidly cool from high temperature | Creates a supersaturated solid solution | Prepares metal for age hardening |
| Trap alloying elements (e.g., copper, silicon) | Prevents undesirable precipitation | Avoids loss of strength and corrosion resistance |
| Freeze unstable crystal structure | Primes material for maximum strength potential | Results in a soft, ductile state (T4 temper) immediately after quenching |
| Balance quench severity | Manages trade-off between strength and distortion | Choice of medium (cold water, polymer, etc.) affects final properties |
Ready to achieve precise heat treatment results with your aluminum alloys? KINTEK specializes in lab equipment and consumables for metallurgical processes, including furnaces and quenching systems tailored for R&D and quality control. Our solutions help you optimize quenching parameters to maximize strength while minimizing distortion. Contact our experts today to discuss how we can support your laboratory's aluminum heat treatment needs and unlock superior material performance.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Brazing Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Molybdenum Vacuum Heat Treat Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the structure of a vacuum furnace? A Guide to Its Core Components & Functions
- What is vacuum furnace high temperature? Unlock the Range for Your Material Processing
- What is the standard thickness of plating? Optimize Durability, Corrosion & Cost
- What are the advantages of a vacuum furnace? Achieve Superior Purity and Control in Heat Treatment
- What is the leak rate for a vacuum furnace? Ensure Process Purity and Repeatability



















