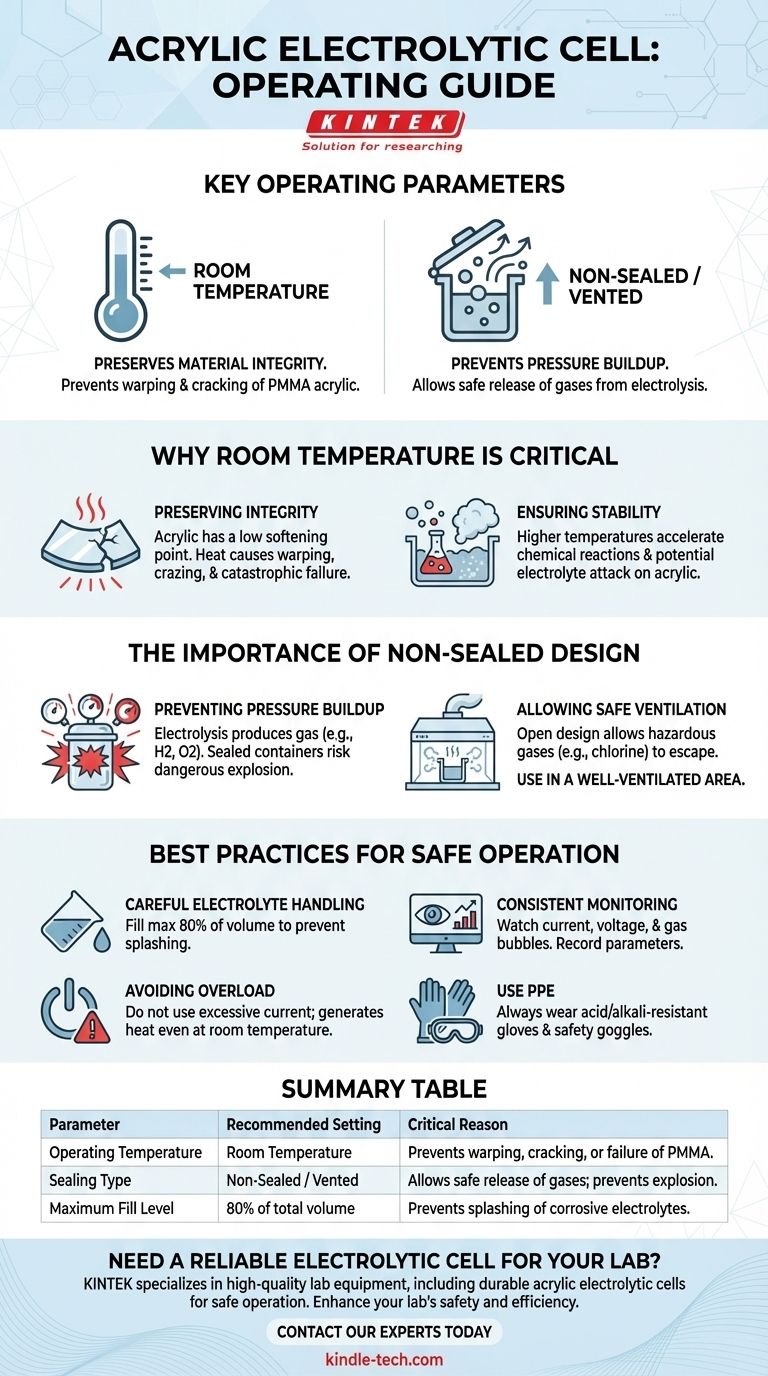
To be clear, the recommended operating temperature for a standard acrylic electrolytic cell is room temperature, and it is designed as a non-sealed piece of equipment. Adhering to these parameters is not merely a suggestion but a critical requirement for both experimental integrity and user safety.
The operational limits of an acrylic cell are dictated by its material properties and the fundamental nature of electrolysis. Operating outside of room temperature risks damaging the cell, while sealing it creates a serious pressure hazard from gas production.

Why Room Temperature is Critical
Operating an acrylic (PMMA) cell at elevated temperatures introduces significant risks to your experiment and the equipment itself. The material is simply not designed for thermal stress.
Preserving Material Integrity
Acrylic has a relatively low softening point compared to laboratory glassware. Exposing it to heat can cause it to warp, craze (form micro-fractures), or lose its structural integrity, leading to leaks and catastrophic failure.
Ensuring Chemical Stability
Higher temperatures can accelerate chemical reactions. This may not only affect your primary electrochemical process but can also increase the rate at which the electrolyte attacks the acrylic, potentially causing clouding or degradation of the cell walls.
The Importance of a Non-Sealed Design
The non-sealed nature of these cells is a fundamental safety feature directly related to the process of electrolysis.
Preventing Dangerous Pressure Buildup
Electrolysis, by definition, breaks down compounds and frequently produces gas. For example, the electrolysis of water generates hydrogen and oxygen gas. In a sealed container, this gas would accumulate, causing a rapid and dangerous increase in pressure that could lead to an explosion.
Allowing for Safe Ventilation
The open or vented design allows hazardous gases, such as chlorine, to escape the cell. This is why it is imperative to operate the cell in a well-ventilated area or a fume hood to prevent the buildup of flammable or toxic fumes in the workspace.
Best Practices for Safe Operation
Proper procedure is essential when working with any electrolytic cell. Following these guidelines ensures safety and the reliability of your results.
Careful Electrolyte Handling
Slowly pour the electrolyte into the cell, never filling it more than 80% of its total volume. This simple step helps prevent splashing of potentially corrosive liquids.
Consistent Monitoring
Once the power is connected, you must monitor the process. Watch for a stable current and voltage, and observe for normal gas bubble formation on the electrodes. Record key parameters like time, temperature, and electrolyte status.
Avoiding Overload
Do not run the cell under prolonged overload conditions. Excessive current can generate significant heat, posing a risk to the acrylic cell even at ambient room temperatures.
Using Personal Protective Equipment (PPE)
Always wear appropriate acid and alkali-resistant gloves and safety goggles. Direct contact with electrolytes can cause severe chemical burns.
Making the Right Choice for Your Goal
Your experimental goals should guide your operational discipline.
- If your primary focus is safety and repeatability: Strictly adhere to room temperature operation and ensure the experiment is conducted in a well-ventilated fume hood.
- If your primary focus is preserving your equipment: Never exceed room temperature and avoid using electrolytes known to be aggressive towards acrylics to ensure the long-term clarity and integrity of the cell.
Ultimately, understanding and respecting the material limitations of your equipment is fundamental to conducting successful and safe electrochemical work.
Summary Table:
| Parameter | Recommended Setting | Critical Reason |
|---|---|---|
| Operating Temperature | Room Temperature | Prevents warping, cracking, or failure of the acrylic material (PMMA). |
| Sealing Type | Non-Sealed / Vented | Allows safe release of gases produced during electrolysis, preventing explosive pressure buildup. |
| Maximum Fill Level | 80% of total volume | Prevents splashing of corrosive electrolytes. |
Need a reliable electrolytic cell for your lab?
Choosing the right equipment is crucial for experimental success and user safety. KINTEK specializes in high-quality lab equipment and consumables, including durable acrylic electrolytic cells designed for safe operation at room temperature.
Let us help you enhance your lab's safety and efficiency. Contact our experts today to find the perfect solution for your electrochemical needs!
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- What is a H type cell? A Guide to Divided Electrochemical Cells for Accurate Experiments
- What optical features does the H-type electrolytic cell have? Precision Quartz Windows for Photoelectrochemistry
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control



















