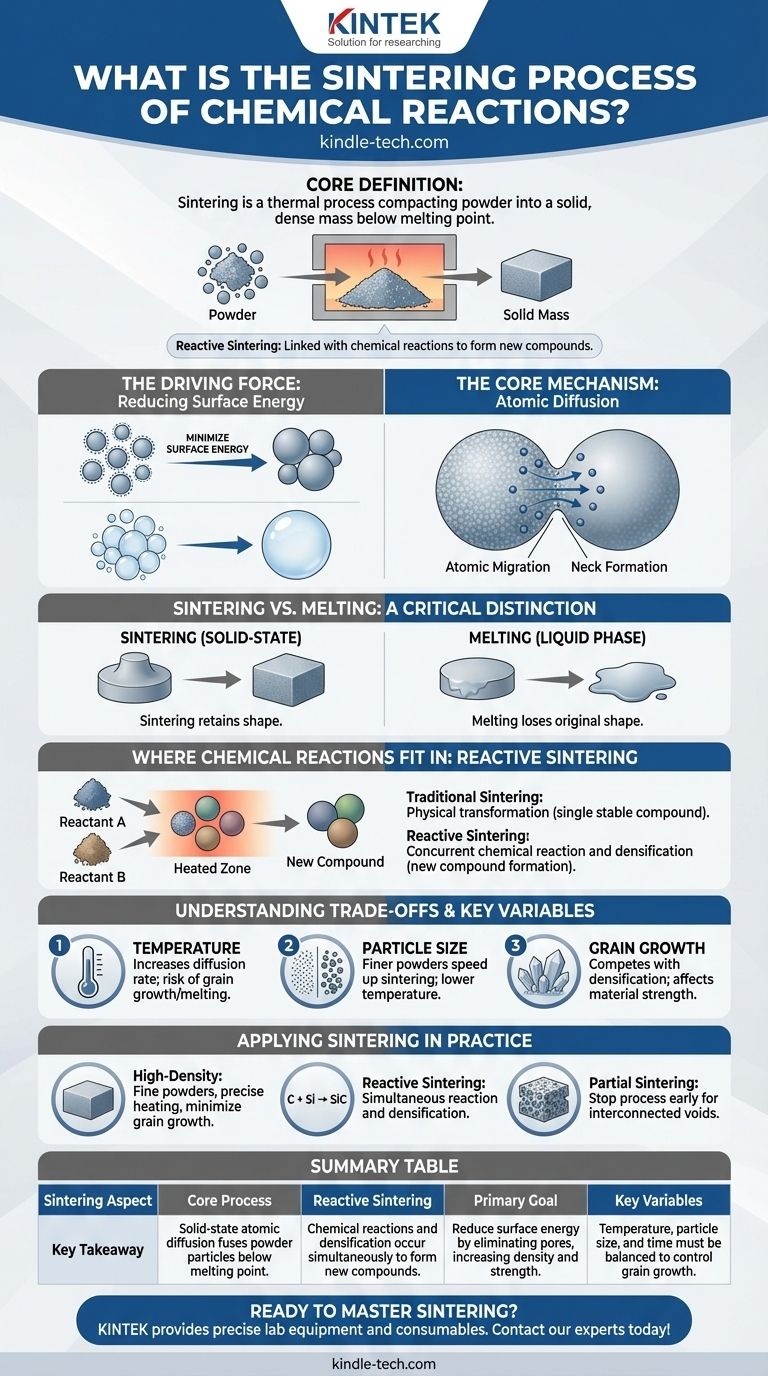
At its core, sintering is a thermal process that compacts a powder into a solid, dense mass using heat and sometimes pressure, all while staying below the material's melting point. While often a purely physical process driven by atomic movement, it can also be intricately linked with chemical reactions to form new compounds in a method known as reactive sintering.
Sintering is not about melting. It is a solid-state phenomenon where atoms migrate across the boundaries of individual particles, fusing them together to reduce surface energy and eliminate the empty spaces between them.

The Fundamental Goal: Why Sintering Happens
The Driving Force: Reducing Surface Energy
A collection of fine powder has an enormous amount of surface area relative to its volume. This high surface area represents a state of high surface energy.
Just as merged soap bubbles have less total surface area than individual ones, nature drives the sintering process to minimize this energy by fusing particles together and reducing the total surface area.
The Core Mechanism: Atomic Diffusion
Sintering happens because heat gives atoms enough energy to move. This process, called atomic diffusion, allows atoms to migrate from one particle to another at their points of contact.
This migration of material gradually builds "necks" between particles. These necks grow over time, pulling the particle centers closer together and systematically eliminating the pores (voids) in the material.
Sintering vs. Melting: A Critical Distinction
Melting is a phase transition where a solid becomes a liquid. This results in a complete loss of the object's original shape.
Sintering, by contrast, occurs entirely in the solid state. This allows a pre-formed object (like one pressed from powder) to become dense and strong while largely retaining its intended geometry.
Where Chemical Reactions Fit In (Reactive Sintering)
Traditional Sintering: A Physical Process
In its most common form, sintering is a physical transformation. A powder of a single, stable compound is heated, and the particles fuse together through the atomic diffusion described above.
Introducing Reactive Sintering
Reactive sintering occurs when the starting material is a mixture of two or more powders that can react with each other. When heated, a chemical reaction and the sintering process happen concurrently.
First, the reactants form a new chemical compound at the particle interfaces. Then, these newly formed product particles sinter together, densifying the material. For example, a mix of silicon and carbon powders can undergo reactive sintering to form dense silicon carbide.
Benefits of Reactive Sintering
This method is powerful for creating advanced materials, like non-oxide ceramics, that are very hard and difficult to process otherwise.
In some cases, the heat generated by an exothermic chemical reaction can even help fuel the sintering process itself, a technique known as combustion synthesis.
Understanding the Trade-offs and Key Variables
The Role of Temperature
Temperature is the primary lever in sintering. Higher temperatures drastically increase the rate of atomic diffusion, accelerating densification.
However, if the temperature is too high, it can lead to undesirable grain growth, which can weaken the final product, or even cause localized melting.
The Impact of Particle Size
Using finer starting powders significantly speeds up sintering. Their higher surface energy provides a stronger driving force for densification to occur at lower temperatures.
The Problem of Grain Growth
As sintering proceeds and pores are eliminated, the individual crystal grains within the material tend to grow larger. This is a competing process with densification.
A successful sintering cycle achieves maximum density while minimizing this grain growth, as overly large grains can make a material brittle. The key is finding the right balance of temperature and time.
Applying Sintering in Practice
Understanding the mechanism allows you to control the outcome based on your objective.
- If your primary focus is creating a high-density ceramic part: You must use fine powders and precisely control the heating cycle to maximize densification before significant grain growth occurs.
- If your primary focus is producing a specific chemical compound: Reactive sintering is your method, where the formation of the new compound and its subsequent densification are coupled.
- If your primary focus is achieving a specific porosity (e.g., for filters): You would intentionally use partial sintering, stopping the process before all pores are eliminated to create an interconnected network of voids.
Mastering the interplay between diffusion, energy, and chemistry is the key to engineering advanced materials from simple powders.
Summary Table:
| Sintering Aspect | Key Takeaway |
|---|---|
| Core Process | Solid-state atomic diffusion fuses powder particles below their melting point. |
| Reactive Sintering | Chemical reactions and densification occur simultaneously to form new compounds. |
| Primary Goal | Reduce surface energy by eliminating pores, increasing density and strength. |
| Key Variables | Temperature, particle size, and time must be balanced to control grain growth. |
Ready to master sintering for your lab's material synthesis needs?
At KINTEK, we specialize in providing the precise lab equipment and consumables required for advanced sintering processes, from high-temperature furnaces to high-purity powders. Whether you are developing new ceramics, optimizing densification cycles, or exploring reactive sintering, our expertise can help you achieve superior results.
Contact our experts today to discuss how we can support your laboratory's specific sintering challenges and accelerate your material development projects.
Visual Guide
Related Products
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Spark Plasma Sintering Furnace SPS Furnace
People Also Ask
- What is one of the newest applications for dental ceramics? Monolithic Zirconia for Full-Arch Bridges
- What is the temperature of sintering zirconia? Mastering the Protocol for Perfect Dental Restorations
- Can you change the color of zirconia crowns? Understanding the Permanent Nature of Zirconia
- What are the white spots on zirconia after sintering? A Guide to Diagnosing and Preventing Defects
- What is the sintering time for zirconia? A Guide to Precise Firing for Optimal Results



















