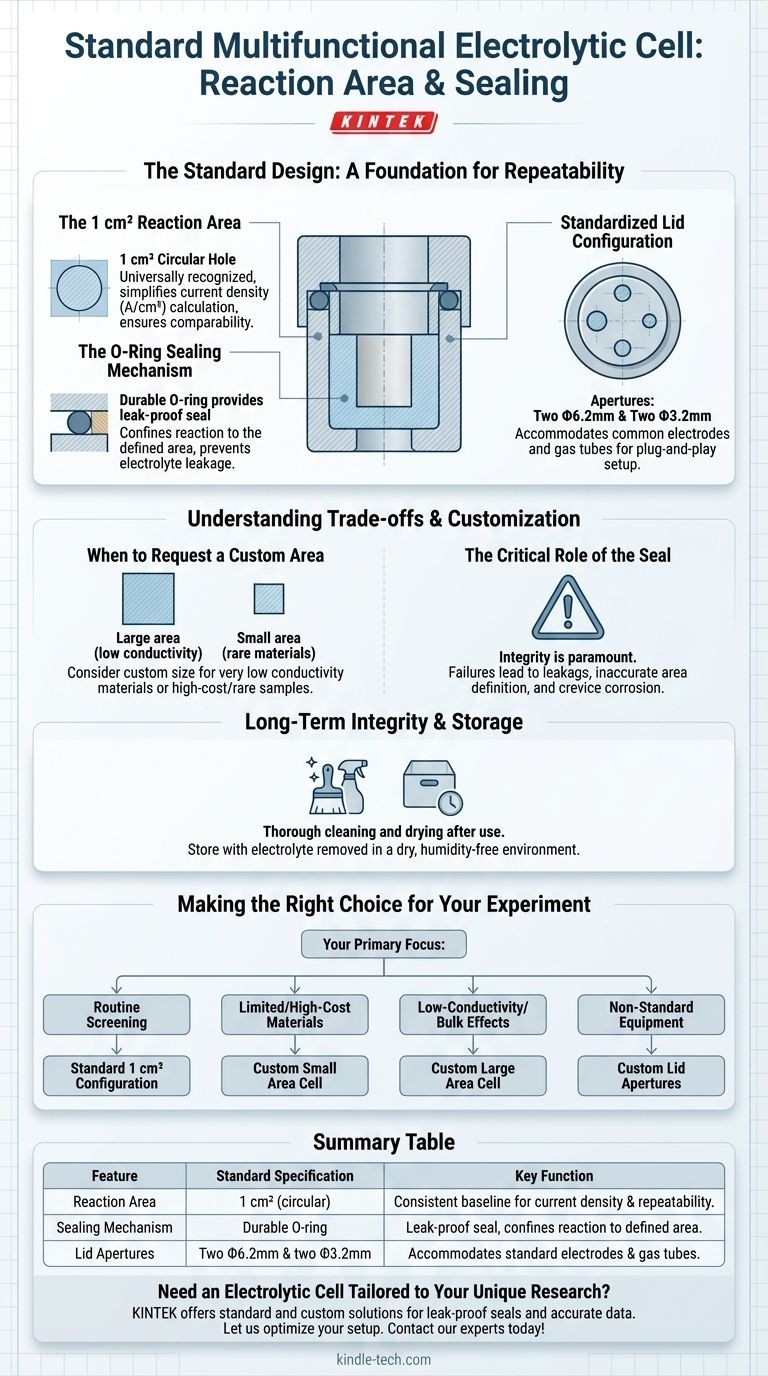
For a standard multifunctional electrolytic cell, the reaction area is a precisely defined circular hole at the bottom of the cell with an area of 1 square centimeter. This area is isolated and made leak-proof by a durable O-ring, which forms a seal between the sample being tested and the main body of the cell.
The standard 1 cm² reaction area, sealed by an O-ring, provides a consistent and reproducible baseline for experiments. The true value of this design lies in its flexibility, as both the reaction area and lid apertures can be customized to fit specific research needs.

The Standard Design: A Foundation for Repeatability
The design of a standard electrolytic cell is centered on creating a controlled and repeatable environment for electrochemical measurements. Every component is chosen to minimize variables and simplify setup.
The 1 cm² Reaction Area
The choice of 1 square centimeter as the standard reaction area is intentional. It serves as a convenient and universally recognized unit, simplifying the calculation of critical metrics like current density (A/cm²).
This standardized area ensures that results can be easily compared across different experiments and even different labs, forming a reliable baseline for material characterization.
The O-Ring Sealing Mechanism
The O-ring is a critical component for ensuring experimental integrity. It is compressed between the main cell body and the working electrode or sample holder.
This compression creates a tight, leak-proof seal. Its primary functions are to prevent the electrolyte from leaking out and, more importantly, to ensure the electrochemical reaction is strictly confined to the defined 1 cm² area.
Standardized Lid Configuration
Beyond the reaction area, the cell lid also follows a standard design. It typically includes several apertures, such as two Φ6.2mm holes and two Φ3.2mm holes.
These standardized openings are designed to accommodate common components like reference electrodes, counter electrodes, and gas sparging tubes, allowing for a quick and secure "plug-and-play" setup.
Understanding the Trade-offs and Customization
While the standard configuration is versatile, it is not universally optimal. Understanding when and why to request custom modifications is key to successful experimentation.
When to Request a Custom Area
The 1 cm² area may not be suitable for all applications. You should consider a custom size if you are working with materials that have very low conductivity (requiring a larger area to generate a measurable signal) or extremely rare or expensive materials (requiring a smaller area to conserve the sample).
The Critical Role of the Seal
Regardless of the area's size, the integrity of the O-ring seal is paramount. A damaged, improperly seated, or chemically degraded O-ring can lead to several critical failures.
These failures include electrolyte leakage, inaccurate definition of the reaction area, and crevice corrosion, all of which will invalidate your experimental data.
Long-Term Integrity and Storage
Proper maintenance is essential for preserving the cell's components, especially the O-ring seal. After use, the cell and electrodes must be thoroughly cleaned and dried.
For long-term storage, the electrolyte should be removed and stored separately. Storing the cell in a dry, humidity-free environment prevents degradation of the sealing components and ensures reliability for future use.
Making the Right Choice for Your Experiment
Selecting the correct cell configuration is the first step toward acquiring accurate and meaningful data. Use your experimental goals to guide your decision.
- If your primary focus is routine material screening or standardized tests: The standard 1 cm² configuration is designed for this purpose and provides excellent comparability.
- If your primary focus is working with limited-quantity or high-cost materials: Request a custom cell with a smaller reaction area to conserve your sample.
- If your primary focus is studying low-conductivity samples or bulk effects: A custom cell with a larger reaction area may be necessary to obtain a sufficient electrochemical signal.
- If your primary focus is integrating non-standard equipment: Ensure you specify custom lid aperture sizes to accommodate your specific electrodes, probes, or sensors.
Understanding these design principles allows you to select or customize an electrolytic cell that ensures the integrity and accuracy of your experimental results.
Summary Table:
| Feature | Standard Specification | Key Function |
|---|---|---|
| Reaction Area | 1 cm² (circular) | Provides a consistent baseline for calculating current density and ensuring repeatable results. |
| Sealing Mechanism | Durable O-ring | Creates a leak-proof seal, confining the reaction to the defined area and preventing electrolyte leakage. |
| Lid Apertures | Two Φ6.2mm & two Φ3.2mm holes | Accommodates standard electrodes (reference, counter) and gas sparging tubes for easy setup. |
Need an Electrolytic Cell Tailored to Your Unique Research?
Whether you require the standard 1 cm² configuration for routine screening or a custom-designed cell for specialized materials (e.g., low-conductivity or high-cost samples), KINTEK has the expertise to deliver. Our precision lab equipment ensures leak-proof seals and reliable performance, critical for accurate electrochemical data.
Let us help you optimize your experimental setup. Contact our experts today to discuss your specific requirements!
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- How should a double-layer water-bath electrolytic cell be operated? A Step-by-Step Guide for Reliable Results
- How should products and waste be handled after an experiment with an H-type electrolytic cell? Ensure Safety and Data Integrity
- What are the design purposes of the stainless steel reactor and the fluoroplastic lid? Ensure High-Purity Electrolysis
- What is the necessity of a non-woven fabric layer in a manganese electrolytic cell? Ensure High-Purity Metal Production
- How should the thin-layer spectroelectrochemical cell be handled to ensure its longevity? Expert Maintenance Tips
- What role does a water-jacketed electrolytic cell play in variable-temperature electrochemical corrosion measurements?
- Process Advantages of Undivided BDD Reactors for Wastewater: Mechanical Simplicity & Dual-Oxidation Efficiency
- Why is PEEK selected for in-situ electrochemical cells in chlor-alkali electrolysis? Superior Chemical Resistance.



















