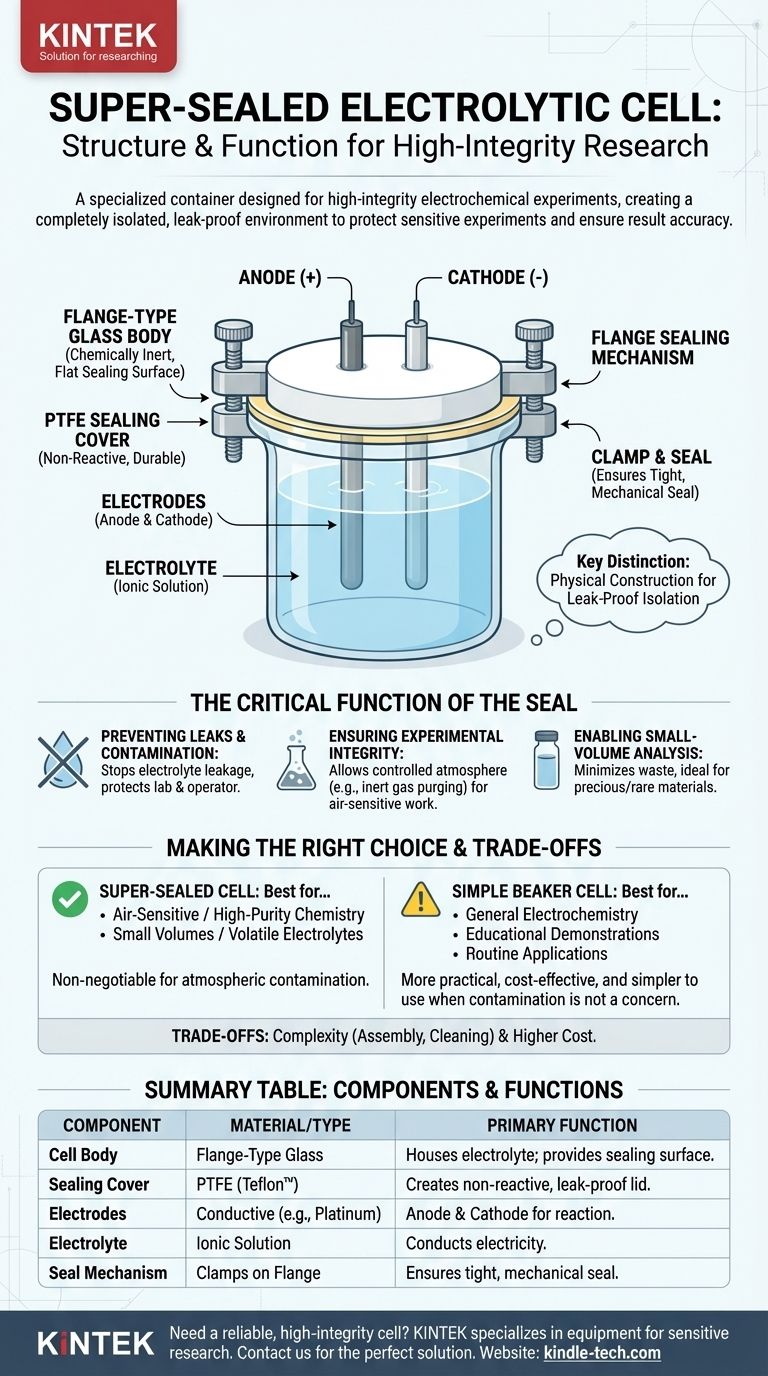
At its core, a super-sealed electrolytic cell is a specialized container designed for high-integrity electrochemical experiments. Its structure combines the fundamental components of any electrolytic cell—electrodes and an electrolyte—with a specialized mechanical sealing system, typically consisting of a flange-type glass cell body and a Polytetrafluoroethylene (PTFE) sealing cover.
The key distinction of a "super-sealed" cell is not its electrochemical function, but its physical construction. The design prioritizes creating a completely isolated, leak-proof environment to protect sensitive experiments from contamination and ensure result accuracy.

Deconstructing the Super-Sealed Cell
To understand this structure, we must separate the universal electrochemical components from the specialized sealing components.
The Core Electrochemical Components
Every electrolytic cell, regardless of its seal, is built around three primary parts.
- Electrodes: These are the two conductive materials, the anode (positive) and the cathode (negative), which are connected to an external power source.
- Electrolyte: This is a fluid solution containing ions, such as dissolved salts in water. The electrolyte acts as the medium that conducts electricity by allowing ions to move freely between the electrodes.
The Specialized Sealing Components
The "super-sealed" designation refers specifically to how the core components are housed.
- Flange-Type Glass Body: The main chamber is made of glass, a chemically inert material. A "flange" is a protruding rim or lip at the top of the cell, which provides a flat, wide surface for creating a strong mechanical seal.
- PTFE Sealing Cover: The lid is made from Polytetrafluoroethylene (PTFE), a highly non-reactive polymer (often known by the brand name Teflon). It resists chemical attack and provides a durable, clean surface.
- The Flange Seal: The PTFE cover is pressed firmly against the glass flange, often secured with clamps. This mechanism ensures a tight, reliable seal that contains the electrolyte and prevents atmospheric gases from entering the cell.
The Critical Function of the Seal
The advanced sealing structure is not arbitrary; it is essential for specific types of electrochemical analysis.
Preventing Leaks and Contamination
A primary function is to prevent any leakage of the electrolyte. Leaks can compromise experimental results, pollute the lab environment, and pose a safety risk to the operator.
Ensuring Experimental Integrity
Many experiments are sensitive to oxygen or moisture in the air. A super-sealed design allows the internal atmosphere to be controlled, for example, by purging it with an inert gas like argon or nitrogen.
Enabling Small-Volume Analysis
The secure and compact design of these cells is ideal for experiments that use a very small amount of solution, minimizing waste and allowing for the study of precious or rare materials.
Understanding the Trade-offs
While offering high performance, the super-sealed design is not universally necessary. Understanding its limitations is key to making an informed choice.
Complexity and Cost
Compared to a simple beaker-style cell, a super-sealed apparatus is more complex to assemble, disassemble, and clean. The precision-machined components also make it significantly more expensive.
When a Simpler Cell Suffices
For many routine or educational applications where atmospheric contamination is not a concern and electrolyte volatility is low, a standard, unsealed glass cell is perfectly adequate and more practical. The "super-sealed" design is a specialized tool for demanding research conditions.
Making the Right Choice for Your Experiment
Selecting the appropriate cell type depends entirely on the requirements of your work.
- If your primary focus is air-sensitive or high-purity chemistry: The super-sealed cell is non-negotiable to prevent atmospheric contamination.
- If your primary focus is working with small volumes or volatile electrolytes: The secure flange seal is essential to prevent sample loss and maintain consistent concentrations.
- If your primary focus is general electrochemistry or educational demonstration: A simpler, unsealed beaker-style cell is often more practical, cost-effective, and easier to use.
Ultimately, choosing the correct electrolytic cell is about matching the tool's capabilities to the demands of your experiment.
Summary Table:
| Component | Material/Type | Primary Function |
|---|---|---|
| Cell Body | Flange-Type Glass | Houses the electrolyte; provides a flat sealing surface. |
| Sealing Cover | PTFE (Teflon™) | Creates a non-reactive, leak-proof lid. |
| Electrodes | Conductive Materials (e.g., Platinum) | Anode and cathode for the electrochemical reaction. |
| Electrolyte | Ionic Solution | Conducts electricity between the electrodes. |
| Seal Mechanism | Clamps on Flange | Ensures a tight, mechanical seal against the glass body. |
Need a reliable, high-integrity cell for your sensitive electrochemical research? KINTEK specializes in high-quality lab equipment, including super-sealed electrolytic cells designed for air-sensitive and small-volume experiments. Our cells ensure the purity and accuracy your work demands. Contact our experts today to find the perfect solution for your laboratory's needs.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
People Also Ask
- What types of electrodes are used in an in-situ Raman electrolytic cell? Optimize for Optical and Electrochemical Control
- How should an all-quartz electrolytic cell and its components be maintained for long-term use? A Guide to Maximizing Equipment Lifespan
- Why is a three-electrode electrochemical cell system standard for corrosion testing? Achieve Precision Measurement
- What system-level maintenance is important for a proton exchange membrane? Ensure Longevity with Proactive System Care
- What core functionality does a three-electrode electrochemical cell provide? Precision Corrosion Testing for Coatings
- How do specialized electrolytic cells facilitate electrochemical testing? Enhance Stainless Steel Corrosion Analysis
- What is the purpose of including a condenser in an electrochemical measurement setup for high-temperature acid solutions?
- What are the key precautions for cleaning the electrolytic cell? Avoid Damage and Ensure Safety



















