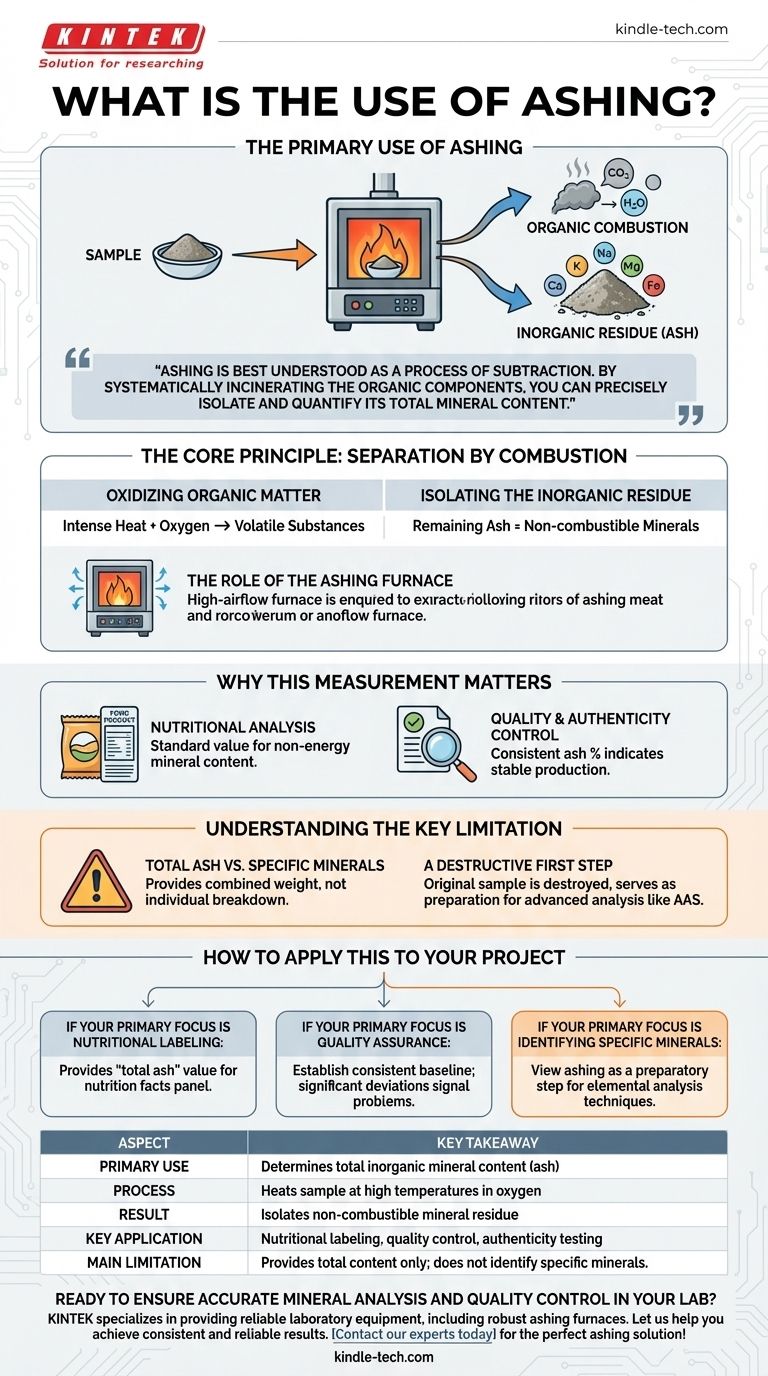
The primary use of ashing is to determine the total amount of inorganic mineral content within a sample. It is a fundamental analytical process where a sample is heated at high temperatures in the presence of oxygen until all the organic matter—such as proteins, fats, and carbohydrates—is burned away. What remains is the incombustible residue, or ash, which represents the sum of all the minerals.
Ashing is best understood as a process of subtraction. By systematically incinerating the organic components of a sample, you can precisely isolate and quantify its total mineral content, a critical measure of quality and nutritional value.

The Core Principle: Separation by Combustion
The ashing process is built on a simple chemical principle: organic compounds combust, while inorganic compounds do not. By controlling this reaction, we can effectively separate these two components.
Oxidizing Organic Matter
A sample is placed in a specialized ashing furnace designed to maintain high temperatures and ensure a steady flow of air. The combination of intense heat and oxygen causes the carbon-based organic compounds to oxidize, breaking down into volatile substances like carbon dioxide and water vapor, which are then vented away.
Isolating the Inorganic Residue
Once the combustion is complete, all the organic material is gone. The substance left behind is the ash—a collection of the inorganic, non-combustible compounds that were originally present in the sample. This residue is primarily composed of various minerals, such as calcium, potassium, sodium, magnesium, and iron.
The Role of the Ashing Furnace
A proper ashing furnace is critical for accurate results. It is engineered to provide a high level of airflow, which not only aids in the complete combustion of the sample but also efficiently removes the smoke and gases created during the process. This ensures that only the pure mineral ash remains.
Why This Measurement Matters
Determining the total ash content is not just an academic exercise; it is a vital metric in many industries, particularly in food science and quality control. The amount of mineral content can reveal a great deal about a product's composition and consistency.
Nutritional Analysis
In the food industry, ashing is a cornerstone of nutritional analysis. The total ash content is a standard value required for nutritional labeling, giving consumers and regulators a clear measure of the food's non-energy-providing mineral components.
Quality and Authenticity Control
For many products, the expected ash content falls within a very specific range. A consistent ash percentage from batch to batch indicates that the raw ingredients and the production process are stable. An unexpected result can signal contamination, adulteration, or a deviation in the formulation.
Understanding the Key Limitation
While incredibly useful, it's crucial to understand what the ashing process does and does not tell you. Misinterpreting the results is a common pitfall.
Total Ash vs. Specific Minerals
The most significant limitation of ashing is that it only provides the total mineral content. It tells you the combined weight of all inorganic compounds but does not differentiate between them.
A Destructive First Step
The process completely destroys the original sample. Furthermore, if you need to know the concentration of a specific mineral, like calcium or iron, ashing is merely the first step. The resulting ash must then be dissolved and analyzed using more advanced techniques, such as atomic absorption spectroscopy.
How to Apply This to Your Project
Your reason for performing an ashing test will determine how you interpret its results.
- If your primary focus is nutritional labeling: Ashing directly provides the "total ash" value required for a complete proximate analysis, which is fundamental to creating an accurate nutrition facts panel.
- If your primary focus is quality assurance: Use ashing to establish a consistent baseline for your product; significant deviations in the ash percentage can signal problems with raw ingredients or processing.
- If your primary focus is identifying specific minerals: View ashing as a preparatory step. The process is necessary to isolate the minerals from the organic matrix before you can proceed with more specific elemental analysis techniques.
Ultimately, ashing transforms a complex sample into a simple, fundamental measure of its inorganic worth.
Summary Table:
| Aspect | Key Takeaway |
|---|---|
| Primary Use | Determines the total inorganic mineral content (ash) of a sample. |
| Process | Heats a sample at high temperatures in oxygen to burn off organic matter. |
| Result | Isolates a residue of non-combustible minerals like calcium, potassium, and iron. |
| Key Application | Nutritional labeling, quality control, and authenticity testing in food and other industries. |
| Main Limitation | Provides total mineral content only; does not identify specific individual minerals. |
Ready to ensure accurate mineral analysis and quality control in your lab?
Ashing is a fundamental step for precise nutritional labeling and quality assurance. KINTEK specializes in providing reliable laboratory equipment, including robust ashing furnaces designed for complete combustion and accurate results. Our products help laboratories in the food, pharmaceutical, and environmental sectors maintain the highest standards of analysis.
Let us help you achieve consistent and reliable results. Contact our experts today to find the perfect ashing solution for your specific needs!
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- Why is an industrial muffle or tube furnace required for CeTe synthesis? Precision Thermal Management for Rare Earths
- What is a box furnace for heat treatment? Versatile Heating Solutions for Industrial Applications
- What is a furnace for ash determination? Unlock Accurate Mineral Content Analysis
- What function does a muffle furnace serve in the preparation of high-purity magnesium? Precision Thermal Control Guide
- How do high-temperature furnaces maintain stability at 300°C? Expert Thermal Control for 304L Stainless Steel
- Why use a constant temperature oven for fluoride ion battery testing? Ensure Precise EIS and CV Data Integrity
- Why is a high-temperature muffle furnace necessary for VO2+ doped nanopowders? Achieve 1000°C Phase Transformation
- What role does a high-temperature muffle furnace play in the fixation of TiO2? Optimize Catalyst Durability & Activity



















