Proper maintenance of a platinum mesh electrode centers on a consistent cycle of cleaning, careful handling, and correct storage. The primary goals are to remove surface impurities that can interfere with electrochemical processes and to preserve the electrode's delicate physical structure. This involves rinsing with deionized water after every use, periodic cleaning with a dilute acid to reactivate the surface, and storing it in a dry, clean environment to prevent contamination and damage.
A platinum mesh electrode is a precision instrument, not just a piece of hardware. Effective maintenance is less about periodic repair and more about a continuous protocol to preserve its catalytic surface integrity and physical structure, ensuring the reliability and repeatability of your results.

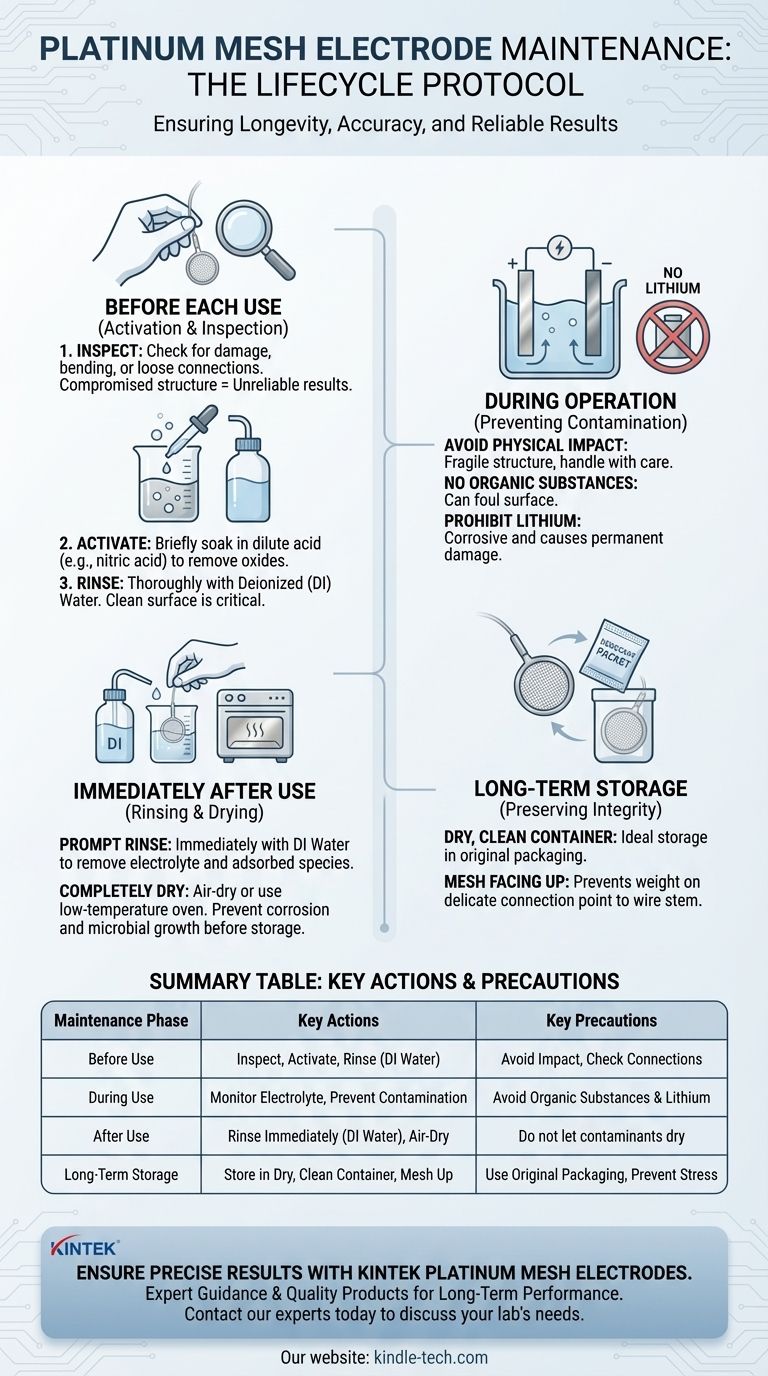
The Electrode Lifecycle: A Maintenance Protocol
To ensure longevity and accuracy, maintenance should not be an occasional event but an integral part of the electrode's use cycle.
Before Each Use: Activation and Inspection
Before starting any experiment, you must prepare the electrode's surface.
First, inspect the electrode for any signs of physical damage, such as bending, tearing in the mesh, or loose wire connections. A compromised structure will produce unreliable results.
Next, activate the platinum surface. This is typically done by briefly soaking the mesh in a dilute acid, such as nitric acid, to remove any surface oxides or organic films acquired during storage.
Finally, rinse the electrode thoroughly with deionized (DI) or distilled water to remove all traces of the acid. A clean, active surface is critical for accurate measurements.
During Operation: Preventing Contamination
While the electrode is in use, your primary goal is to prevent irreversible contamination or damage.
The platinum mesh is soft and mechanically fragile. Avoid any physical impact or pressure that could bend or deform the mesh structure.
Be acutely aware of your electrolyte composition. Avoid contact with organic substances that can foul the surface and are difficult to remove.
Most critically, prohibit any use with lithium ions. Lithium is corrosive to platinum and can cause permanent damage to the electrode.
Immediately After Use: Rinsing and Drying
Prompt cleaning after an experiment is essential to prevent reactants from drying onto and fouling the electrode surface.
Immediately remove the electrode from the electrolyte solution and rinse it thoroughly with deionized water. This removes residual electrolyte and loosely adsorbed species.
After rinsing, allow the electrode to air-dry or place it in a low-temperature oven. Ensure it is completely dry before storage to prevent corrosion or microbial growth.
Long-Term Storage: Preserving Integrity
Proper storage protects the electrode from both physical damage and environmental contamination.
Store the dried electrode in a dry, clean, and non-contaminating container. Its original packaging is often the ideal choice.
When placing it in storage, ensure the platinum mesh faces upwards. This prevents the delicate connection point between the mesh and the wire stem from bearing any weight, which is a common point of failure.
Understanding Common Pitfalls
Even with a proper protocol, certain risks can compromise your electrode's performance and lifespan. Awareness is key to prevention.
The Risk of Mechanical Damage
The high surface area of a platinum mesh comes at the cost of structural fragility. It is easily bent, stretched, or torn.
Some guides suggest polishing solid disk electrodes with alumina powder to restore the surface. Do not attempt this with a mesh electrode. Abrasive polishing will destroy the fine wires of the mesh.
Always check that wire connections are secure. A loose connection introduces high resistance and will invalidate your electrochemical data.
The Danger of Chemical Contamination
Contamination is the most common cause of performance degradation. It occurs when molecules irreversibly adsorb to the platinum surface, blocking active sites.
Fouling by organic compounds or polymers can be difficult to reverse with simple acid washing. If you know your experiment may produce these, consider using a different, less valuable electrode.
As noted, lithium is particularly destructive. However, other metallic ions and certain sulfur or phosphorus compounds can also poison the platinum surface. Always review your chemistry beforehand.
Recognizing Performance Degradation
Over time, you may notice a decline in performance, such as drifting potentials or reduced current density. This indicates the electrode surface is fouled or damaged.
If routine acid washing does not restore performance, the contamination may be severe. If you observe visible damage, scratches, or significant discoloration that cannot be cleaned, the electrode should be replaced to ensure data integrity.
How to Apply This to Your Work
Your maintenance strategy should align with your experimental goals and tolerance for error.
- If your primary focus is maximizing electrode lifespan: Prioritize gentle handling and immediate post-use cleaning with deionized water. Store it meticulously in its original box to prevent physical stress.
- If your primary focus is ensuring the highest data accuracy: Implement a strict pre-use acid wash and DI water rinse protocol every single time. Regularly inspect the electrode under magnification for micro-fouling or damage.
- If you work with "dirty" or unknown systems: Dedicate a specific, lower-cost electrode for exploratory work to avoid contaminating your high-precision reference electrodes.
By treating your platinum mesh electrode as the sensitive instrument it is, you will ensure it remains a reliable tool for discovery.
Summary Table:
| Maintenance Phase | Key Actions | Key Precautions |
|---|---|---|
| Before Use | Inspect for damage, activate with dilute acid, rinse with DI water | Avoid physical impact, check wire connections |
| During Use | Monitor electrolyte, prevent contamination | Avoid organic substances and lithium ions |
| After Use | Rinse immediately with DI water, air-dry completely | Do not let contaminants dry on the surface |
| Long-Term Storage | Store in a dry, clean container with mesh facing up | Use original packaging to prevent stress on connections |
Ensure your electrochemical experiments are precise and repeatable with KINTEK's high-quality platinum mesh electrodes and expert support.
As a leading supplier of laboratory equipment and consumables, KINTEK understands that the integrity of your electrodes is critical to your research outcomes. Our platinum mesh electrodes are designed for durability and performance, but proper maintenance is key to maximizing their lifespan.
Let us help you achieve reliable results:
- Expert Guidance: Our team can provide detailed maintenance protocols tailored to your specific application.
- Quality Products: Access electrodes that meet the highest standards for catalytic surface integrity.
- Ongoing Support: Get advice on handling, storage, and troubleshooting to prevent common pitfalls like contamination or mechanical damage.
Don't let electrode maintenance uncertainties compromise your data. Contact our experts today to discuss your lab's needs and discover how KINTEK's solutions can enhance your workflow and ensure long-term electrode performance.
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
People Also Ask
- What are the performance characteristics of platinum sheet electrodes? Unlock Superior Electrochemical Performance
- What is the proper post-treatment procedure for a platinum sheet electrode? Ensure Long-Term Accuracy & Protect Your Investment
- What are the available specifications for platinum sheet electrodes? Find the Perfect Fit for Your Electrochemical Needs
- How should a platinum sheet electrode be pretreated before use? Ensure Accurate Electrochemical Measurements
- What is the expected lifespan of a platinum sheet electrode? Maximize Your Electrode's Service Life











