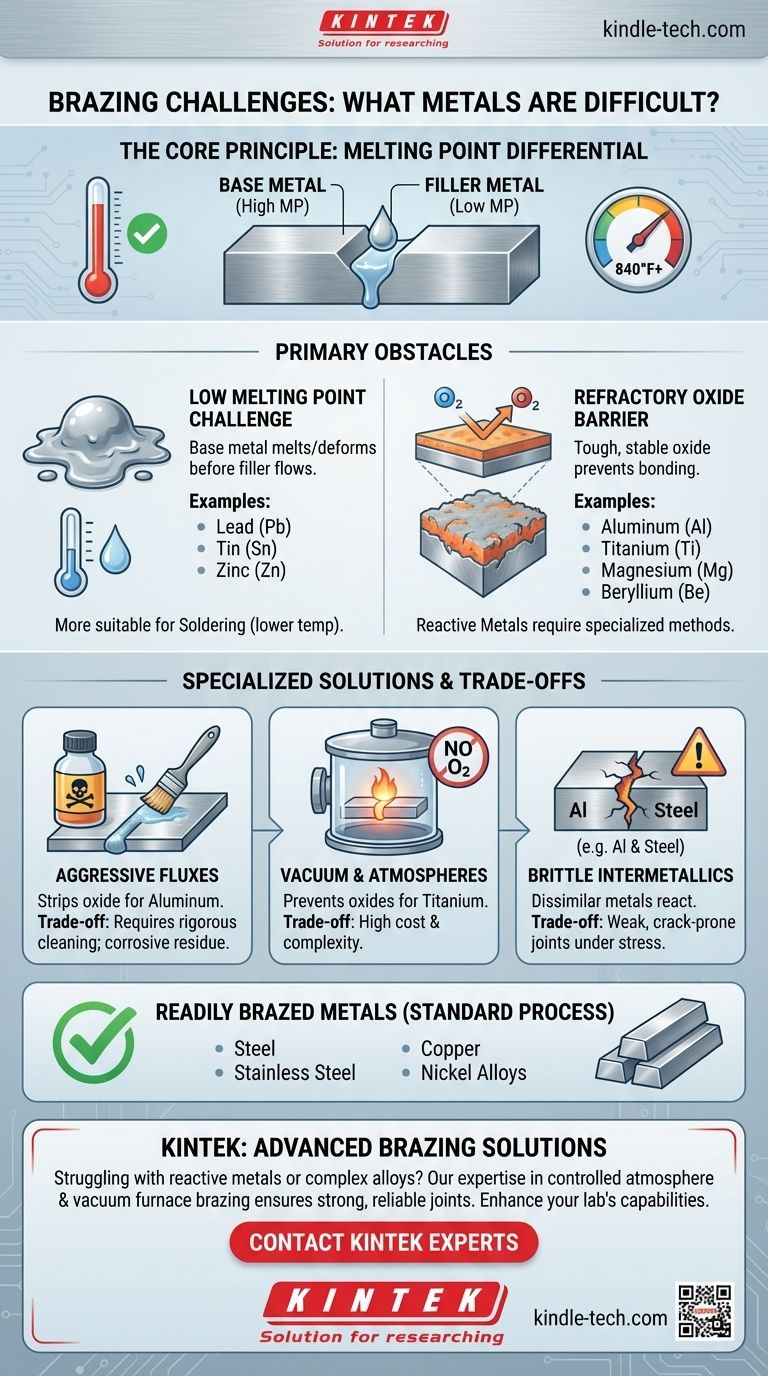
While brazing is a remarkably versatile process, no metal is fundamentally impossible to join. Instead, certain metals present significant challenges that make them impractical or impossible to braze using standard techniques. The primary obstacles are a very low melting point that is close to the brazing temperature or the formation of a tough, stable oxide layer that prevents the filler metal from bonding.
The question is not which metals cannot be brazed, but rather what metallurgical properties—like low melting points or tenacious oxides—make a metal extremely difficult to braze without highly specialized processes, fluxes, or controlled atmospheres.

The Core Principle of Brazing
To understand the challenges, we must first revisit the fundamental requirement of brazing. The process works by heating two base metals and introducing a filler metal that has a lower melting point. This filler metal melts, is drawn into the joint by capillary action, and then solidifies, creating a strong metallurgical bond.
H3: The Temperature Differential is Key
For a successful braze, the melting point of the base metals must be significantly higher than the melting point of the filler metal. This allows the filler to become fully liquid and flow properly without melting or damaging the parts being joined.
Metals That Challenge the Brazing Process
The metals that are considered difficult or "unbrazeable" typically fall into two main categories.
H3: The Low Melting Point Challenge
If a base metal's melting point is too close to the brazing temperature range (typically 840°F / 450°C and higher), the base metal itself will begin to melt or deform.
This effectively makes standard brazing impossible for metals like lead, tin, and zinc. These materials are more suitable for soldering, which uses much lower-temperature filler metals.
H3: The Refractory Oxide Barrier
Many highly useful metals react instantly with oxygen in the air to form a tough, stable, and self-healing layer of oxide on their surface. This oxide layer acts as a barrier, preventing the liquid braze filler from "wetting" or bonding with the pure base metal beneath.
Metals known for this challenge include:
- Aluminum
- Magnesium
- Titanium
- Beryllium
These are often called reactive metals. While they can be brazed, the process requires overcoming this oxide layer, which complicates the operation significantly.
Understanding the Trade-offs and Solutions
Brazing challenging metals is not impossible, but it demands specialized techniques that come with significant trade-offs in cost, complexity, and post-processing.
H3: Aggressive Fluxes
For metals like aluminum, a highly active and often corrosive flux is required. This chemical agent aggressively strips the oxide layer away just ahead of the flowing filler metal. The major trade-off is that these flux residues must be meticulously cleaned from the assembly after brazing to prevent future corrosion.
H3: Vacuum and Controlled Atmospheres
For extremely reactive metals like titanium, even the most aggressive flux is insufficient. The only reliable method is to perform the brazing operation inside a vacuum furnace. By removing all oxygen, the formation of the oxide layer is prevented entirely, allowing the filler metal to bond directly to the base metal. This process produces exceptionally clean and strong joints but is far more expensive and complex than open-air brazing.
H3: The Risk of Brittle Intermetallics
When brazing dissimilar metals, particularly reactive metals to common alloys like steel (e.g., aluminum to steel), a new problem can arise. At brazing temperatures, the two distinct metals can react with each other at the joint interface, forming hard and brittle intermetallic compounds. These compounds can severely weaken the joint, making it prone to cracking under stress.
Making the Right Choice for Your Application
In contrast to the challenges above, metals like steel, stainless steel, copper, and nickel alloys are readily brazed because their surface oxides are easily removed by standard fluxes, making them ideal for a wide range of applications.
- If your primary focus is simplicity and cost-effectiveness: Choose common base metals like steel, copper, or brass, which are easily brazed with standard equipment and fluxes.
- If your primary focus is joining lightweight aluminum: Be prepared to use specialized aluminum brazing alloys and highly active fluxes that require rigorous post-braze cleaning procedures.
- If your primary focus is high-performance titanium or reactive metals: You must plan for advanced and costly processes like vacuum furnace brazing to ensure joint integrity.
Ultimately, understanding these material challenges transforms brazing from a simple task into a precise engineering process.
Summary Table:
| Metal Category | Key Challenge | Common Examples |
|---|---|---|
| Low Melting Point Metals | Melting point too close to brazing temperature | Lead, Tin, Zinc |
| Reactive Metals | Forms tough, stable oxide layer | Aluminum, Titanium, Magnesium |
Struggling to join challenging metals like aluminum or titanium? KINTEK specializes in advanced brazing solutions and lab equipment for reactive metals and complex alloys. Our expertise in controlled atmosphere and vacuum furnace brazing ensures strong, reliable joints for your most demanding applications. Contact our experts today to discuss your specific metal joining needs and discover how we can enhance your laboratory's capabilities!
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What is the cost of a vacuum brazing furnace? A guide to key factors and investment strategy
- What is vacuum brazing? The Ultimate Guide to High-Purity, Flux-Free Metal Joining
- Where are vacuum furnaces used? Essential for High-Purity Heat Treatment in Critical Industries
- What is the basic of brazing? A Guide to Strong, Low-Heat Metal Joining
- Why would you braze instead of weld? Preserve Material Integrity and Join Dissimilar Metals



















