At its core, sintering is the process that transforms a fragile compact of ceramic powder into a strong, dense, solid object. This transformation is driven by heat and involves several critical physical changes, primarily atomic diffusion that leads to the formation of bonds between particles, overall shrinkage of the component, and a significant reduction in internal porosity.
Sintering is fundamentally a process of reducing the total surface energy of a powder system. By applying heat, you give atoms the mobility to move, closing the gaps between particles to create a dense, low-energy final structure. Understanding this driving force is the key to controlling the outcome.

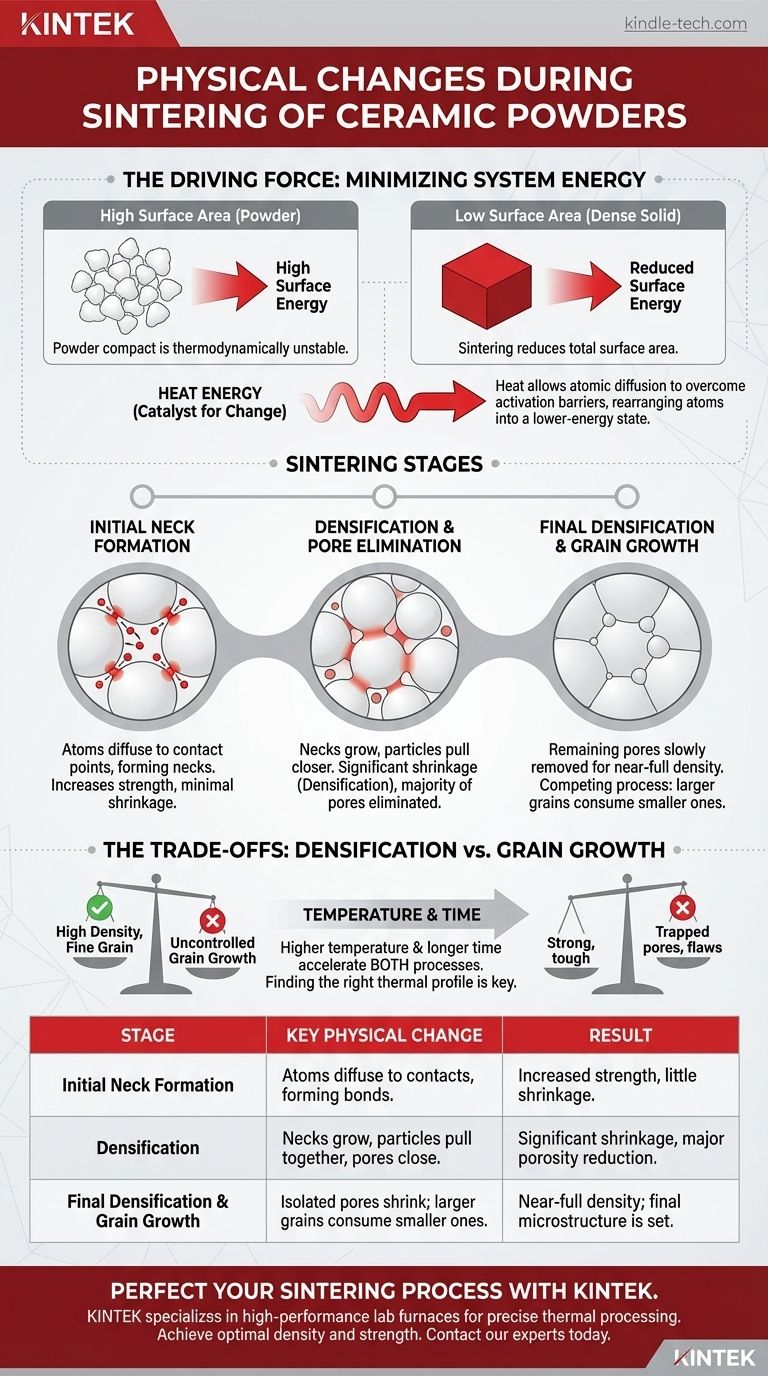
The Driving Force: Minimizing System Energy
Before examining the physical changes, it's crucial to understand why they happen. The answer lies in thermodynamics.
The Problem of High Surface Area
A collection of fine powder particles has an immense amount of surface area compared to a single solid block of the same mass. This vast surface represents a state of high surface energy.
Just as water droplets merge to reduce their total surface area, a powder compact is in a thermodynamically unstable state. The system naturally wants to reduce this excess energy.
Heat as the Catalyst for Change
Sintering provides the necessary energy, in the form of heat, to overcome the activation barriers for atomic movement. This thermal energy allows atoms to move, or diffuse, from one location to another.
This atomic diffusion is the fundamental mechanism responsible for all the macroscopic physical changes observed during the process. The entire goal of the system is to rearrange itself into a lower-energy state, which means eliminating surfaces and the pores between them.
The Key Physical Transformations During Sintering
Sintering is not a single event but a continuous process typically viewed in three overlapping stages.
Stage 1: Initial Neck Formation
As the powder compact heats up, the first significant change is the formation of "necks" at the contact points between adjacent particles.
Atoms diffuse to these contact points, creating small bridges that begin to bond the particles together. This increases the component's strength but results in very little densification or shrinkage at this stage.
Stage 2: Densification and Pore Elimination
This is the most critical stage for achieving a dense final product. The necks between particles grow substantially, pulling the centers of the particles closer together.
This collective movement causes the entire component to shrink, a change known as densification. The network of interconnected pores from the initial compact begins to close off, forming isolated, spherical pores. The vast majority of porosity is eliminated during this intermediate stage.
Stage 3: Final Densification and Grain Growth
In the final stage, the remaining isolated pores are slowly eliminated, leading to the last few percent of densification. This process is much slower because it's harder for vacancies (the absence of atoms) to diffuse out of the isolated pores to a free surface.
Simultaneously, a competing process called grain growth becomes dominant. To further reduce system energy, larger grains begin to consume smaller ones, reducing the total area of grain boundaries.
Understanding the Trade-offs: Densification vs. Grain Growth
Successfully sintering a ceramic is a balancing act between achieving full density and controlling the final grain size, as these two phenomena are often in competition.
The Goal: High Density, Fine Grain Structure
For most structural applications, the ideal ceramic has near-100% density and a fine, uniform grain structure. High density eliminates weak points, while small grains generally lead to higher strength and fracture toughness.
The Problem with Uncontrolled Grain Growth
If grain growth occurs too rapidly, it can be detrimental. Fast-moving grain boundaries can sweep past pores, trapping them inside the grains.
Once a pore is trapped within a grain, it is extremely difficult to remove, effectively halting densification and leaving permanent flaws in the material.
The Temperature and Time Dilemma
Higher temperatures and longer sintering times accelerate all diffusion processes. This speeds up densification but also dramatically accelerates grain growth.
This creates the central challenge of sintering: finding a thermal profile (heating rate, temperature, and hold time) that maximizes the rate of densification while minimizing the rate of grain growth.
Optimizing Sintering for Your Desired Outcome
The ideal sintering parameters depend entirely on the properties you want to achieve in the final component.
- If your primary focus is maximum mechanical strength: You must prioritize achieving near-full density while keeping the final grain size as small as possible, which may require advanced methods like pressure-assisted sintering or the use of grain growth inhibitors.
- If your primary focus is optical transparency: You must eliminate virtually all porosity, as pores scatter light. This often requires sintering in the final stage for longer times, even at the expense of some grain growth, to ensure all pores are removed.
- If your primary focus is rapid, cost-effective production: You will likely use higher sintering temperatures to reduce cycle time, accepting a compromise in the form of a larger final grain size that may slightly reduce peak mechanical performance.
Mastering the art of sintering is about precisely controlling the movement of atoms to engineer a material's microstructure from the ground up.
Summary Table:
| Sintering Stage | Key Physical Change | Result |
|---|---|---|
| Initial Neck Formation | Atoms diffuse to particle contacts, forming bonds. | Increased strength, little shrinkage. |
| Densification | Necks grow, particles pull together, pores close. | Significant shrinkage, major porosity reduction. |
| Final Densification & Grain Growth | Isolated pores shrink; larger grains consume smaller ones. | Near-full density; final microstructure is set. |
Ready to perfect your ceramic sintering process and achieve optimal density and strength?
KINTEK specializes in high-performance lab furnaces and consumables for precise thermal processing. Whether you're developing advanced ceramics for structural, optical, or electronic applications, our equipment delivers the controlled heating profiles essential for mastering the delicate balance between densification and grain growth.
Contact our thermal processing experts today to discuss how our solutions can help you engineer superior ceramic components.
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Does ceramic break with heat? The Real Culprit is Thermal Shock
- Why the melting temperature of ceramic is higher than for most metals? Unpacking Atomic Bond Strength
- Why ceramics can withstand high temperature? Unlock the Secrets of Atomic Structure
- How long is the calcination process? Optimize Your Process Time for Maximum Efficiency
- What is dry ashing in a muffle furnace? A Guide to Precise Mineral Analysis



















