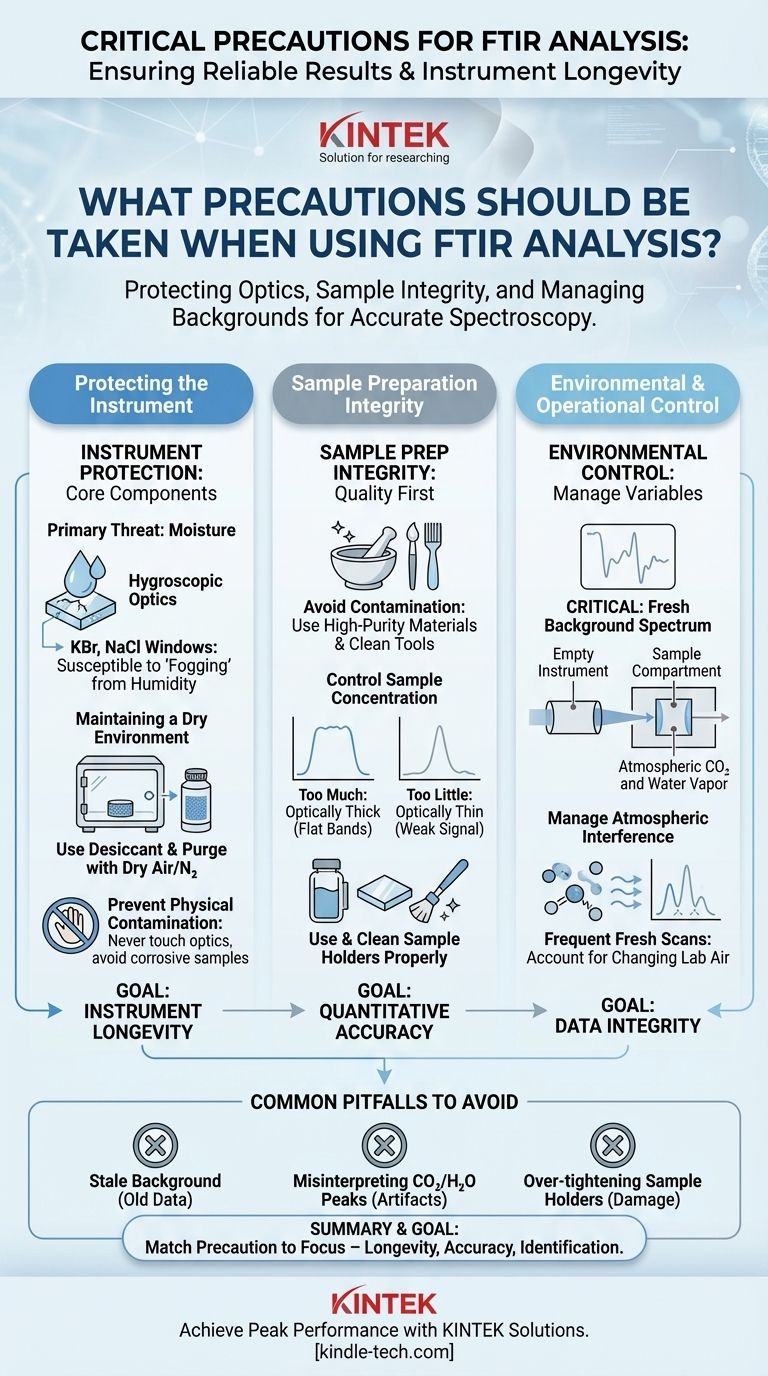
To ensure reliable results and protect the instrument, the most critical precautions for FTIR analysis revolve around three areas: protecting the sensitive optics from moisture and contamination, meticulous sample preparation to avoid interference, and correctly managing the atmospheric background. These steps are not merely procedural; they are fundamental to acquiring accurate and reproducible spectroscopic data.
The central challenge in FTIR is managing environmental variables. Your primary precautions should focus on protecting the instrument's hygroscopic optics from humidity and compensating for atmospheric CO2 and water vapor through proper background scans.

Protecting the Instrument's Core Components
An FTIR spectrometer is a precision instrument with sensitive, and often expensive, internal components. Protecting them is your first priority.
The Primary Threat: Moisture
The biggest enemy of a standard FTIR instrument is moisture. Many of the optical components, like the beamsplitter and windows, are made from hygroscopic (water-absorbing) materials such as potassium bromide (KBr) or sodium chloride (NaCl).
Exposing these optics to humidity in the air can cause them to become cloudy or "fogged" over time, permanently degrading the instrument's performance and requiring costly replacement.
Maintaining a Dry Environment
To combat moisture, the optical bench is typically sealed and contains a desiccant, such as silica gel. You must monitor this desiccant and regenerate or replace it when it becomes saturated (often indicated by a color change).
For high-performance applications, the instrument should be purged with a continuous flow of dry air or nitrogen gas to displace any moisture and CO2 from the beam path.
Preventing Physical Contamination
Never touch optical components with your bare hands. Fingerprints will leave an oily residue that can permanently damage coatings and absorb infrared light, distorting your spectra.
Similarly, ensure that no part of your sample, especially corrosive liquids or fine powders, comes into direct contact with the instrument's internal mirrors or windows.
Ensuring Sample Preparation Integrity
The quality of your spectrum is directly determined by the quality of your sample preparation. Inaccurate results are more often caused by poor sample handling than instrument malfunction.
Avoid Contamination at All Costs
Use only clean spatulas, mortars and pestles, and glassware. When preparing KBr pellets, use high-purity, spectroscopy-grade KBr, as standard lab-grade KBr can contain water and other impurities.
If using solvents, ensure they are also high-purity and do not have absorption bands in the region of interest.
Control Sample Concentration
The amount of sample is critical. Too much sample (optically thick) will cause your primary absorption bands to be completely flat ("totally absorbing"), hiding valuable information.
Too little sample (optically thin) will result in a weak signal with a poor signal-to-noise ratio, making small peaks impossible to distinguish from baseline noise.
Use and Clean Sample Holders Properly
Whether you are using salt plates for thin films, a liquid cell, or an Attenuated Total Reflectance (ATR) accessory, ensure it is appropriate for your sample and meticulously cleaned before and after use. Residue from a previous analysis is a common source of spectral contamination.
Understanding Environmental and Operational Precautions
What happens outside and inside the sample compartment has a profound impact on your final spectrum.
The Critical Role of the Background Spectrum
An FTIR does not measure the sample's absorbance directly. It first measures a background spectrum (of the empty instrument) and then a sample spectrum. It then automatically ratios these two to produce the final absorbance spectrum.
This background scan accounts for the instrument's state and, most importantly, for any infrared-active gases in the atmosphere, namely water vapor and carbon dioxide (CO2).
Manage Atmospheric Interference
Because the background and sample scans are performed at different times, any change in the lab's atmosphere can lead to poor subtraction. This results in sharp, characteristic peaks from atmospheric water vapor (around 3600-3900 cm⁻¹ and 1300-1900 cm⁻¹) and CO2 (around 2360 cm⁻¹ and 667 cm⁻¹) appearing in your final spectrum.
You must run a fresh background scan frequently, especially if the lab environment changes (e.g., doors opening, changes in humidity).
Common Pitfalls to Avoid
Even with the best intentions, several common mistakes can compromise your data. Being aware of them is key to troubleshooting your results.
Using a "Stale" Background
The most common error is using a background spectrum that was collected hours or even days before. The atmosphere in a lab is dynamic. Always collect a fresh background immediately before running your sample for the most accurate results.
Misinterpreting Atmospheric Peaks
Beginners often mistake the sharp, rotational-vibrational peaks of CO2 or water vapor for features of their sample. Learning to recognize the distinct appearance of these atmospheric artifacts is a crucial skill.
Over-tightening Sample Holders
When using an ATR accessory or a pellet press, there is a temptation to apply excessive force. This can crack or permanently indent the expensive crystal (like diamond or zinc selenide), leading to poor results and costly repairs. Apply only enough pressure to ensure good contact.
Making the Right Choice for Your Goal
Your approach to these precautions depends on your primary objective.
- If your primary focus is instrument longevity: Make protecting the hygroscopic optics from moisture your absolute priority by managing desiccants and using a purge gas.
- If your primary focus is quantitative accuracy: Meticulous sample preparation and collecting a fresh background spectrum immediately before every sample are non-negotiable.
- If your primary focus is qualitative identification: Learn to recognize and ignore the tell-tale spectral artifacts from atmospheric CO2 and water vapor so they don't confuse your interpretation.
Ultimately, adopting these precautions will ensure the long-term health of your instrument and give you confidence in the integrity of every spectrum you collect.
Summary Table:
| Precaution Area | Key Action | Primary Goal |
|---|---|---|
| Instrument Protection | Maintain dry environment with desiccant/purge gas; avoid touching optics. | Instrument Longevity |
| Sample Preparation | Use clean, high-purity materials; control sample concentration. | Quantitative Accuracy |
| Environmental Control | Collect a fresh background scan before each sample. | Data Integrity |
| Common Pitfalls | Recognize atmospheric CO2/water vapor peaks; avoid over-tightening holders. | Accurate Interpretation |
Achieve Peak Performance with Your FTIR Analysis
Ensuring the accuracy of your spectroscopic data and the longevity of your equipment requires precision and the right support. KINTEK specializes in providing high-quality lab equipment and consumables, including spectroscopy-grade materials and accessories, to meet the exacting demands of your laboratory.
Let our experts help you optimize your FTIR processes. From selecting the right desiccants and purge gases to choosing the perfect sample holders, we are here to support your research and quality control.
Contact our team today to discuss your specific needs and discover how KINTEK can enhance the reliability and efficiency of your laboratory operations.
Visual Guide
Related Products
- Laboratory Test Sieves and Sieving Machines
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Benchtop Laboratory Vacuum Freeze Dryer
People Also Ask
- How are vibratory sieve shakers and standard sieves utilized to analyze the effects of biomass torrefaction? Optimize Grindability
- Why is a precision vibrating sieve shaker essential for metal leaching research? Optimize Your Particle Size Analysis
- How is a vibratory sieve shaker used in the particle size analysis of mechanically alloyed powders? Expert Guide
- What is the function of sieving equipment in CuAlMn alloys? Master Pore Size Precision
- What is the primary purpose of using standard sieves? Master Particle Uniformity for High-Quality Catalyst Preparation



















