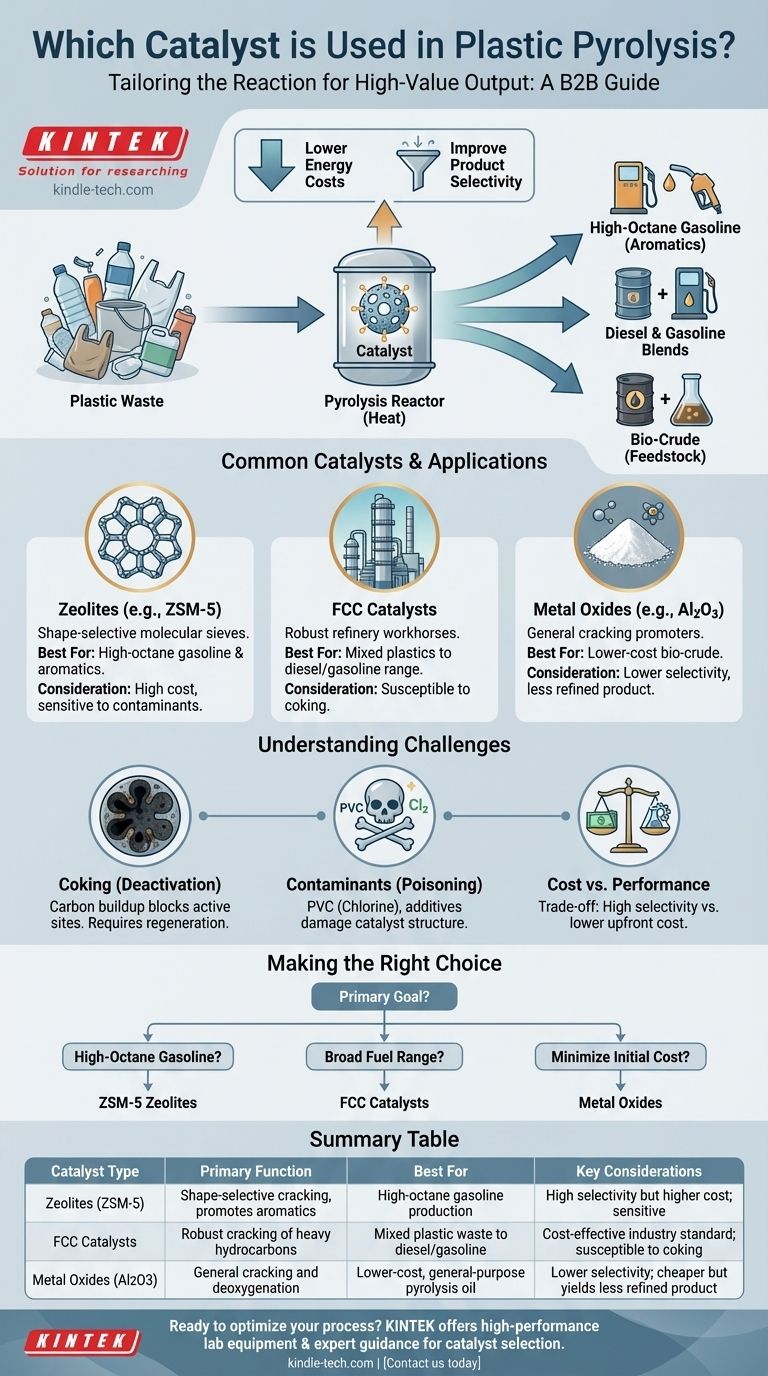
There is no single catalyst used for plastic pyrolysis. Instead, the choice depends entirely on the desired end-product, with the most common and effective options being zeolite-based catalysts (like ZSM-5), Fluid Catalytic Cracking (FCC) catalysts borrowed from the oil industry, and various metal oxides. These catalysts are critical for lowering the process temperature and selectively guiding the chemical reactions to produce high-value fuels and chemical feedstocks.
The central challenge in plastic pyrolysis is not merely breaking down plastic, but precisely controlling how it breaks down. The choice of catalyst is the primary tool for steering this process, determining whether the output is a valuable gasoline blend, diesel fuel, or a feedstock for new chemicals.

The Core Function of a Catalyst in Pyrolysis
A catalyst's role extends far beyond simply speeding up a reaction. In the context of converting plastic waste to oil, it serves two primary, economically critical functions: lowering energy costs and improving product quality.
Lowering Activation Energy
Pyrolysis breaks the long polymer chains of plastics into smaller, more useful hydrocarbon molecules. This process requires a significant amount of energy (heat).
A catalyst provides an alternative chemical pathway for this breakdown—one that requires much less energy. This allows the pyrolysis reactor to operate at a lower temperature, substantially reducing fuel consumption and operational costs.
Enhancing Product Selectivity
Without a catalyst, pyrolysis is an uncontrolled thermal cracking process that produces a wide, unpredictable range of molecules, including low-value char and non-condensable gases.
A catalyst offers a structured surface with specific active sites that favor certain reactions. This "selectivity" guides the cracking process to produce a narrower, more desirable range of hydrocarbons, such as those found in gasoline or diesel.
Common Catalysts and Their Applications
The selection of a catalyst is a strategic decision based on the type of plastic feedstock and the target product.
Zeolites (e.g., ZSM-5, HZSM-5)
Zeolites are crystalline aluminosilicates with a highly ordered, porous structure. Think of them as "molecular sieves" with pores of a specific size.
Their shape-selective nature makes them exceptionally good at producing aromatic hydrocarbons, which are high-octane components ideal for gasoline blending. ZSM-5 is the most widely studied and effective catalyst for this purpose.
Fluid Catalytic Cracking (FCC) Catalysts
These are the workhorses of traditional oil refineries, designed to crack heavy crude oil fractions into gasoline.
Because of their proven effectiveness and relative low cost, spent or equilibrium FCC catalysts are often used in plastic pyrolysis. They are excellent for cracking mixed plastic waste into a broad spectrum of liquid fuels, including gasoline and diesel-range hydrocarbons.
Metal Oxides
Simple metal oxides like silica (SiO2), alumina (Al2O3), and titania (TiO2) can also be used as catalysts.
While generally less selective than zeolites, they are effective at promoting cracking and are often cheaper. They are typically used when the goal is a less-refined pyrolysis oil (bio-crude) that will undergo further upgrading.
Understanding the Trade-offs and Challenges
No catalyst is a perfect solution. Real-world implementation involves navigating significant operational and economic challenges.
Catalyst Deactivation by Coking
During pyrolysis, a carbonaceous residue known as coke inevitably deposits onto the catalyst's surface.
This coke blocks the active sites and pores, rendering the catalyst progressively less effective over time. This deactivation requires a costly and energy-intensive regeneration step (burning off the coke) or complete replacement of the catalyst.
Sensitivity to Contaminants
Plastic waste streams are rarely pure. Contaminants can quickly poison a catalyst.
Plastics like PVC release chlorine, which is highly corrosive and deactivates many catalysts. Similarly, elements present in additives and dyes can permanently damage the catalyst's structure, reducing its lifespan and efficiency.
Cost vs. Performance
There is a direct trade-off between the cost of a catalyst and its performance.
Highly selective, custom-designed zeolites can produce high-quality fuel fractions but come at a significant cost. In contrast, cheaper options like basic metal oxides or spent FCC catalysts lower upfront investment but may yield a lower-quality product that requires more expensive post-processing.
Making the Right Choice for Your Goal
The optimal catalyst is defined by the specific objective of the pyrolysis operation.
- If your primary focus is high-octane gasoline production: The superior shape selectivity of ZSM-5 zeolites is the most effective choice.
- If your primary focus is a broad range of liquid fuels (diesel and gasoline): Robust and cost-effective FCC catalysts are the industry standard for processing mixed plastics.
- If your primary focus is minimizing initial cost for general-purpose cracking: Basic metal oxides or a non-catalytic thermal process may be the most viable starting point.
Ultimately, the catalyst is the critical component that transforms plastic pyrolysis from a crude disposal method into a sophisticated chemical recycling process.
Summary Table:
| Catalyst Type | Primary Function | Best For | Key Considerations |
|---|---|---|---|
| Zeolites (e.g., ZSM-5) | Shape-selective cracking, promotes aromatics | High-octane gasoline production | High selectivity but higher cost; sensitive to contaminants |
| FCC Catalysts | Robust cracking of heavy hydrocarbons | Mixed plastic waste to diesel/gasoline | Cost-effective industry standard; susceptible to coking |
| Metal Oxides (e.g., Al2O3) | General cracking and deoxygenation | Lower-cost, general-purpose pyrolysis oil | Lower selectivity; cheaper but yields less refined product |
Ready to optimize your plastic pyrolysis process? The right catalyst is critical for maximizing fuel yield and quality. At KINTEK, we specialize in providing high-performance laboratory equipment and consumables tailored to your R&D and production needs. Whether you're testing catalyst efficiency or scaling up your operation, our experts can help you select the perfect tools for success. Contact us today to discuss how KINTEK can support your laboratory and pyrolysis goals!
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Special Shape Press Mold for Lab
- Zirconia Ceramic Gasket Insulating Engineering Advanced Fine Ceramics
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Precision Machined Zirconia Ceramic Ball for Engineering Advanced Fine Ceramics
People Also Ask
- What are the advantages of metal sintering? Achieve Cost-Effective, Complex Metal Parts
- What is the pressure less sintering method? Achieve Complex Shapes Without High-Pressure Equipment
- What is a mixed hot zone and what is its primary disadvantage? Understanding Contamination Risks
- What is the significance of maintaining a high vacuum environment during the sintering of ODS iron-based alloys?
- Why do superdry reforming processes require high-temperature furnaces? Unlock Higher Conversion with Precision Control
- What is the primary role of a high-precision laboratory oven in the solvothermal synthesis of Cu-BTC? Drive MOF Quality
- What is the temperature of a vacuum furnace? Unlock the Right Range for Your Process
- Does annealing make steel stronger? Discover the True Purpose of This Heat Treatment



















