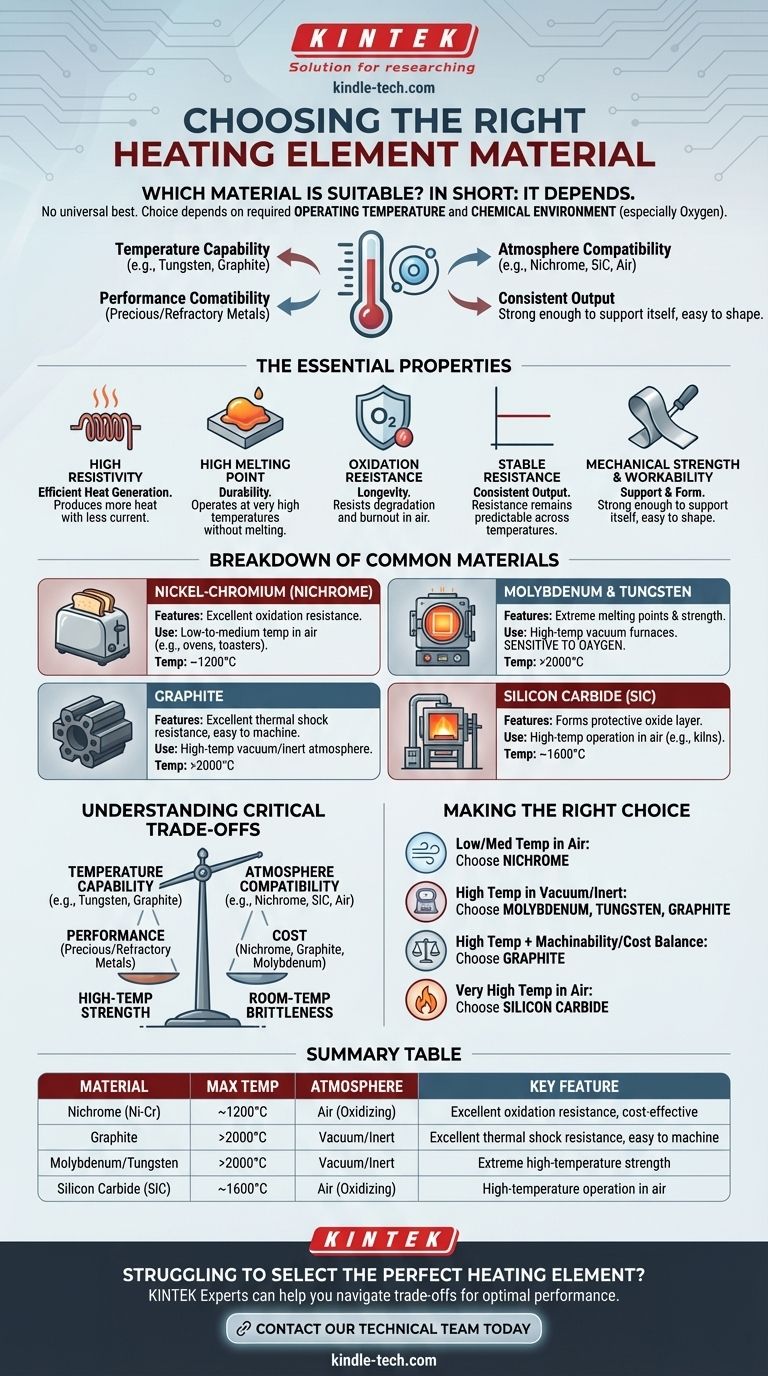
In short, the most suitable materials for heating elements are specialized alloys like Nichrome (nickel-chromium), refractory metals such as molybdenum and tungsten, and non-metallic compounds like graphite and silicon carbide. The best choice is not universal; it depends entirely on the required operating temperature and the chemical environment, particularly the presence of oxygen.
The core principle is not finding a single "best" material, but rather matching a material's specific properties—its melting point, resistivity, and resistance to oxidation—to the precise demands of your application.

The Essential Properties of a Heating Element
To understand why certain materials are chosen, we must first look at the ideal characteristics required for converting electricity into heat reliably and efficiently.
High Resistivity for Efficient Heat Generation
A heating element works by resisting the flow of electricity, which generates heat (Joule heating). A material with high electrical resistivity will produce a significant amount of heat with less current, making the system more efficient.
High Melting Point for Durability
The material must operate at very high temperatures without melting or deforming. A high melting point is a non-negotiable property that ensures the element's structural integrity and long-term stability.
Resistance to Oxidation for Longevity
Many applications operate in air. At high temperatures, oxygen aggressively attacks most materials, causing them to degrade and fail. A suitable material must be "free from oxidation" or form a protective oxide layer to ensure a reasonable service life.
Stable Resistance for Consistent Output
As an element heats up, its resistance can change. A low temperature coefficient of resistance is crucial because it ensures the material's resistance—and therefore its heat output—remains stable and predictable across its operating temperature range.
Mechanical Strength and Workability
The material must be strong enough to support its own weight at high temperatures without sagging or breaking. It also needs to be workable enough to be formed into practical shapes like coils or ribbons, a property known as good machinability.
A Breakdown of Common Materials
Heating element materials fall into distinct categories, each suited for different environments and temperature ranges.
Nickel-Chromium Alloys (Nichrome)
These are the go-to materials for common, low-to-medium temperature applications like toasters, space heaters, and industrial ovens. Their key advantage is excellent oxidation resistance, allowing them to operate for long periods in open air.
Molybdenum & Tungsten
These are refractory metals known for their extremely high melting points and strength at high temperatures. They are popular choices for high-temperature vacuum furnaces. However, they are highly sensitive to oxygen and will rapidly burn out if operated in air at high temperatures.
Graphite
Graphite is a non-metal that is exceptional for high-temperature vacuum or inert atmosphere applications. It is prized for its high-temperature resistance, excellent thermal shock resistance, and superior machinability, making it easy to form into complex shapes.
Silicon Carbide (SiC)
Like Nichrome, silicon carbide can be used at high temperatures in an oxygen-rich atmosphere. It forms a protective layer of silicon dioxide that prevents further oxidation, making it suitable for applications like industrial kilns and furnaces that operate in air.
Understanding the Critical Trade-offs
Choosing a material is always a balancing act. The ideal choice for one application may be a catastrophic failure in another.
Temperature vs. Atmosphere
This is the single most important trade-off. Materials with the highest temperature capabilities, like tungsten, molybdenum, and graphite, cannot be used in the presence of oxygen. Materials that can operate in air, like Nichrome and silicon carbide, have lower maximum operating temperatures.
Performance vs. Cost
Precious metals like platinum and refractory metals like tantalum offer incredible performance at very high temperatures but come at a significant cost. For most applications, materials like molybdenum, graphite, and nickel-chromium alloys provide a much better balance of performance and affordability.
High-Temp Strength vs. Room-Temp Brittleness
Some materials that are strong and ductile at high temperatures can be brittle and difficult to handle at room temperature. This can impact the manufacturing, installation, and maintenance of the heating element.
Making the Right Choice for Your Application
Your final decision should be guided by the specific goals and constraints of your system.
- If your primary focus is low-to-medium temperature heating in air: Nickel-chromium (Nichrome) alloys are the standard due to their unmatched oxidation resistance and cost-effectiveness.
- If your primary focus is high-temperature heating in a vacuum or inert gas: Molybdenum, tungsten, and graphite are superior choices, offering high strength at extreme temperatures where oxygen is absent.
- If your primary focus is balancing high-temperature performance with machinability and cost: Graphite is often the most practical and versatile solution for vacuum furnace applications.
- If your primary focus is very high-temperature heating in air: Silicon carbide is the ideal material, as it can withstand intense heat without degrading from oxidation.
Ultimately, selecting the right heating element is a process of aligning the material's inherent characteristics with the specific environmental and thermal demands of your system.
Summary Table:
| Material | Max Temperature | Atmosphere | Key Feature |
|---|---|---|---|
| Nichrome (Ni-Cr) | ~1200°C | Air (Oxidizing) | Excellent oxidation resistance, cost-effective |
| Graphite | >2000°C | Vacuum/Inert | Excellent thermal shock resistance, easy to machine |
| Molybdenum/Tungsten | >2000°C | Vacuum/Inert | Extreme high-temperature strength |
| Silicon Carbide (SiC) | ~1600°C | Air (Oxidizing) | High-temperature operation in air |
Struggling to select the perfect heating element material for your lab furnace or oven?
At KINTEK, we specialize in lab equipment and consumables. Our experts can help you navigate the critical trade-offs between temperature, atmosphere, and material properties to ensure optimal performance, longevity, and cost-efficiency for your specific application—whether you need robust Nichrome for an air atmosphere or high-purity graphite for a vacuum environment.
Contact our technical team today for a personalized consultation to enhance your lab's heating capabilities.
Visual Guide
Related Products
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Infrared Heating Quantitative Flat Plate Press Mold
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
People Also Ask
- How does precision temperature-controlled heating equipment facilitate Cu3N to MCL conversion? Optimize MCL Synthesis
- What is the most accurate temperature sensor? Why RTDs Lead in Precision and Stability
- Do heating elements use a lot of electricity? Understanding High Energy Consumption and Efficient Alternatives
- Why are high-performance sealing and refractory materials critical for high-temperature solar thermochemical reactors?
- Why are high-power electric heating rods used in in-situ catalyst reaction cells? Ensure Precision & Thermal Stability
- Is tungsten shock resistant? Uncovering the Surprising Brittleness of a Hard Metal
- What is the best material for high temperature furnace elements to be used in oxidizing atmospheres? Choose the Right Element for Your Lab
- Which is better quartz or ceramic heaters? The ultimate guide to spot vs. space heating.
















