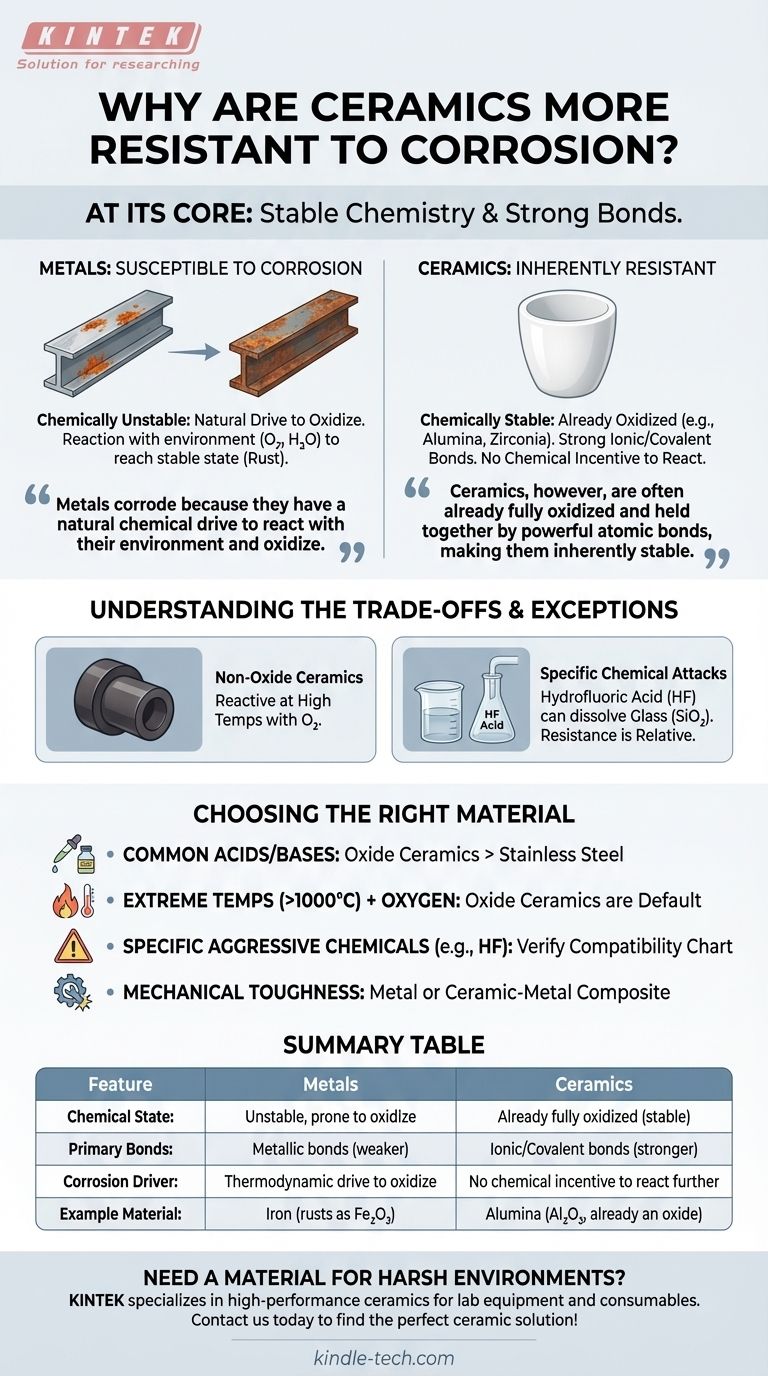
At its core, a ceramic's resistance to corrosion stems from its fundamental chemistry. Most ceramics are compounds formed from metallic and non-metallic elements, held together by incredibly strong ionic or covalent bonds. This structure means they are often already in their most stable, oxidized state, leaving very little chemical incentive for them to react further with their environment. Unlike metals that corrode by oxidizing, most advanced ceramics have effectively already "corroded" to their final, most stable form.
Metals corrode because they have a natural chemical drive to react with their environment and oxidize. Ceramics, however, are often already fully oxidized and held together by powerful atomic bonds, making them inherently stable and non-reactive in most corrosive environments.

The Chemical Nature of Corrosion: A Tale of Two Materials
To understand why ceramics are so stable, it is best to compare them directly to metals, which are defined by their susceptibility to corrosion.
How Metals Corrode: The Drive to Oxidize
Metals in their pure, usable form (like an iron beam or an aluminum sheet) are in a chemically unstable state. They have a strong thermodynamic drive to react with oxygen, water, or other elements in their environment.
This reaction, called oxidation, allows the metal to reach a lower, more stable energy state. The result is a new compound, such as iron oxide (rust). Corrosion is simply the visible outcome of a metal's natural tendency to return to its more stable, oxidized form.
Why Ceramics Resist: The Stability of Oxides
Many of the most common and robust technical ceramics—such as alumina (aluminum oxide, Al₂O₃) and zirconia (zirconium dioxide, ZrO₂)—are already oxides. They are the very compounds that metals become after corroding completely.
Because they are already in their highest oxidation state, there is no further chemical gain for them to be had by reacting with oxygen. You cannot "rust" a material that is, chemically speaking, already rust.
The Power of Strong Bonds
The atoms in a ceramic are typically linked by ionic and covalent bonds. These are extremely strong, rigid connections that require a significant amount of energy to break.
For a chemical to corrode a ceramic, it must have enough energy to sever these powerful bonds. Most common acids and bases simply lack the ability to do so, leaving the ceramic's surface unaffected. This is in stark contrast to the weaker metallic bonds in metals, which allow atoms to be stripped away more easily.
Understanding the Trade-offs and Exceptions
While exceptionally resistant, ceramics are not invincible. Their performance depends on the specific ceramic and the specific corrosive agent.
The Exception of Non-Oxide Ceramics
Not all ceramics are oxides. Materials like silicon carbide (SiC) or silicon nitride (Si₃N₄) are highly valued for their hardness and performance at extreme temperatures.
However, because they are not fully oxidized, they can still react with oxygen at very high temperatures. This is still a form of corrosive degradation, though it typically occurs under conditions far more extreme than those that would destroy most metals.
Chemical Attack on the Atomic Structure
Certain highly aggressive chemicals can break down even the most stable ceramics. The classic example is glass (amorphous silicon dioxide, SiO₂), a type of ceramic known for its excellent chemical resistance.
However, hydrofluoric acid (HF) will readily dissolve glass. The fluoride ion has a unique and powerful affinity for silicon, allowing it to break the strong silicon-oxygen bonds and form new, stable silicon-fluorine compounds. This demonstrates that corrosion resistance is relative, not absolute.
The Role of Grain Boundaries
Most ceramics are polycrystalline, meaning they are composed of many tiny crystal grains packed together. The boundaries between these grains can be points of structural weakness or can collect impurities during manufacturing.
Corrosive agents can sometimes exploit these grain boundaries, initiating corrosion there even when the grains themselves are resistant. This is a primary focus of advanced ceramic engineering—to create purer, denser microstructures with fewer weak points.
Choosing the Right Material for Your Application
Your material choice depends entirely on the specific environmental threats you need to mitigate. Understanding a ceramic's inherent chemical stability allows you to deploy it where it offers a decisive advantage.
- If your primary focus is resisting common acids, bases, and saltwater: Most oxide ceramics like alumina or zirconia offer superior and more reliable performance than even high-grade stainless steels.
- If you face extremely high temperatures (over 1000°C) with oxygen present: An oxide ceramic is the default choice, as even specialized superalloys will rapidly oxidize and fail, whereas the ceramic remains stable.
- If your environment contains specific, highly aggressive chemicals like hydrofluoric acid: You must verify the ceramic's specific chemical compatibility chart, as general rules of resistance may not apply.
- If mechanical toughness and resistance to sudden fracture are paramount: A metal or a ceramic-metal composite is often a better choice, as pure ceramics are inherently brittle despite their hardness and corrosion resistance.
By understanding that a ceramic's strength comes from its inherent chemical stability, you can confidently select it for the environments it was born to withstand.
Summary Table:
| Feature | Metals | Ceramics |
|---|---|---|
| Chemical State | Unstable, prone to oxidize | Already fully oxidized (stable) |
| Primary Bonds | Metallic bonds (weaker) | Ionic/Covalent bonds (stronger) |
| Corrosion Driver | Thermodynamic drive to oxidize | No chemical incentive to react further |
| Example Material | Iron (rusts as Fe₂O₃) | Alumina (Al₂O₃, already an oxide) |
Need a material that can withstand harsh chemicals and extreme temperatures? KINTEK specializes in high-performance lab equipment and consumables made from advanced ceramics like alumina and zirconia, designed for superior corrosion resistance and long-term reliability in demanding laboratory environments. Contact us today to find the perfect ceramic solution for your specific application!
Visual Guide
Related Products
- Engineering Advanced Fine Alumina Al2O3 Ceramic Rod Insulated for Industrial Applications
- Zirconia Ceramic Gasket Insulating Engineering Advanced Fine Ceramics
- Silicon Carbide (SIC) Ceramic Sheet Wear-Resistant Engineering Advanced Fine Ceramics
- Precision Machined Zirconia Ceramic Ball for Engineering Advanced Fine Ceramics
- Advanced Engineering Fine Ceramics Boron Nitride (BN) Ceramic Parts
People Also Ask
- Why is high-purity alumina (Al2O3) preferred over quartz for steam oxidation? Ensure Data Integrity at 1773 K
- Is silicon carbide used in high temperature applications? Master Extreme Heat with SiC
- Does silicon carbide have high thermal conductivity? Unlock Superior Heat Management for Demanding Applications
- Is there anything better than a ceramic coating? Yes, for ultimate paint protection, combine PPF & ceramic coatings.
- What is the process of sintering in ceramic materials? A Guide to Transforming Powder into High-Strength Parts
- What are the 4 main classes of ceramic materials? A Guide to Their Functions and Applications
- What is the maximum operating temperature of alumina? The Critical Role of Purity and Form
- Does ceramic break with temperature change? The Critical Role of Thermal Shock Explained

















