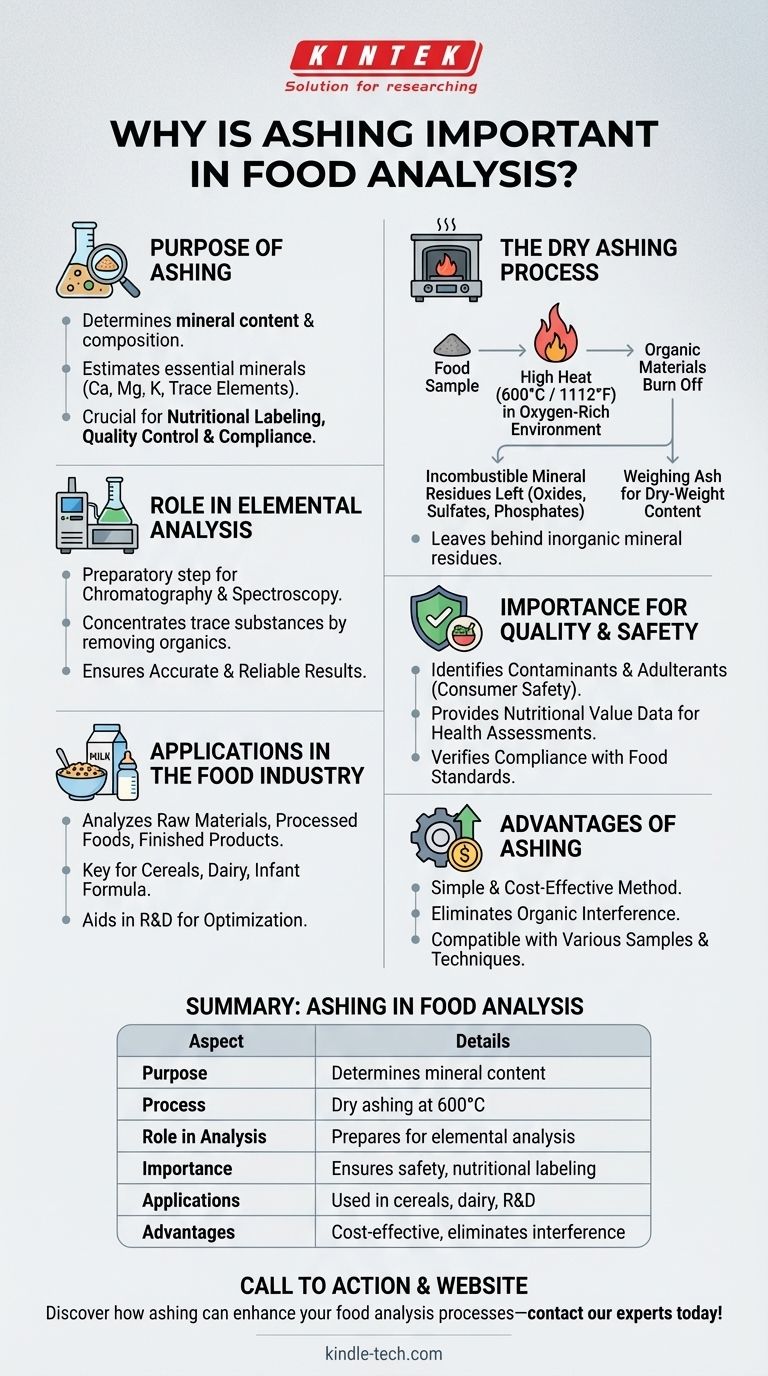
Ashing is a critical process in food analysis, primarily used to determine the mineral content and composition of food samples. By heating the sample to high temperatures (around 600°C) in an oxygen-rich environment, organic materials are burned off, leaving behind incombustible mineral residues such as oxides and sulfates. This process, known as dry ashing, serves as a preliminary step for further elemental analysis, enabling accurate estimation of mineral concentrations based on dry-weight ash content. Ashing is essential for ensuring food quality, safety, and compliance with regulatory standards, as it provides valuable insights into the nutritional and mineral profile of food products.

Key Points Explained:
-
Purpose of Ashing in Food Analysis:
- Ashing is primarily used to determine the mineral content of food samples.
- It helps in estimating the concentration of essential minerals like calcium, magnesium, potassium, and trace elements.
- The process is crucial for nutritional labeling, quality control, and ensuring compliance with food safety regulations.
-
Dry Ashing Process:
- Dry ashing involves heating the food sample in an ashing furnace at high temperatures (around 600°C or 1112°F) in the presence of oxygen.
- Organic materials are combusted, leaving behind inorganic mineral residues such as oxides, sulfates, and phosphates.
- The remaining ash is weighed to determine the mineral content based on the dry weight of the sample.
-
Role in Elemental Analysis:
- Ashing serves as a preparatory step for further elemental analysis techniques like chromatography or spectroscopy.
- By removing organic compounds, the process concentrates trace substances, making it easier to analyze the mineral composition.
- This ensures accurate and reliable results in subsequent analytical procedures.
-
Importance for Food Quality and Safety:
- Ashing helps identify contaminants or adulterants in food products, ensuring consumer safety.
- It provides data on the nutritional value of food, which is essential for dietary planning and health assessments.
- Regulatory bodies often require ash content analysis to verify compliance with food standards.
-
Applications in the Food Industry:
- Ashing is widely used in the food industry to analyze the mineral content of raw materials, processed foods, and finished products.
- It is particularly important for products like cereals, dairy, and infant formula, where mineral content is a key quality parameter.
- The process also aids in research and development, helping food scientists optimize formulations and improve product quality.
-
Advantages of Ashing:
- Provides a simple and cost-effective method for mineral analysis.
- Eliminates organic interference, ensuring accurate measurement of inorganic components.
- Compatible with a wide range of food samples and analytical techniques.
In summary, ashing is a fundamental technique in food analysis that enables the determination of mineral content, supports quality control, and ensures regulatory compliance. Its role in preparing samples for further elemental analysis makes it indispensable in both research and industrial applications.
Summary Table:
| Aspect | Details |
|---|---|
| Purpose | Determines mineral content and composition of food samples. |
| Process | Dry ashing at 600°C burns organic materials, leaving inorganic residues. |
| Role in Analysis | Prepares samples for elemental analysis like chromatography and spectroscopy. |
| Importance | Ensures food safety, nutritional labeling, and regulatory compliance. |
| Applications | Used in cereals, dairy, infant formula, and R&D for quality optimization. |
| Advantages | Cost-effective, eliminates organic interference, and compatible with many techniques. |
Discover how ashing can enhance your food analysis processes—contact our experts today!
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- How do high-temperature furnaces ensure the accuracy of Inconel oxidation testing? Achieve Stable Thermal Environments
- What is a box furnace used for? A Versatile Tool for Heat Treatment, Sintering & Analysis
- What are the advantages of dry ashing over wet ashing? Streamline Your Lab's Sample Prep
- What is the principle of muffle furnace in laboratory? Master Precise High-Temp Heating
- Why is precise temperature control in laboratory ovens critical for photocatalytic pigments? Protect Color & Function
- How does an industrial-grade ageing furnace enhance Cu-Cr alloys? Optimize Strength and Conductivity
- What is the role of the high-temperature muffle furnace and cooling system in simulating thermal fatigue?
- Why is a high-temperature muffle furnace used for Ga-LLZO calcination? Master Your Solid-State Synthesis



















