In materials science, carbon coating is a critical surface modification technique used to significantly boost the performance and longevity of active materials, especially within lithium-ion batteries. It acts as a multi-functional layer that simultaneously improves electrical conductivity, provides a protective chemical barrier, and reinforces the material's physical structure.
The core value of carbon coating is not simply adding a layer, but engineering a solution that solves three distinct problems at once: poor conductivity, chemical instability, and structural failure. It transforms inherently flawed but promising materials into robust, high-performance components.
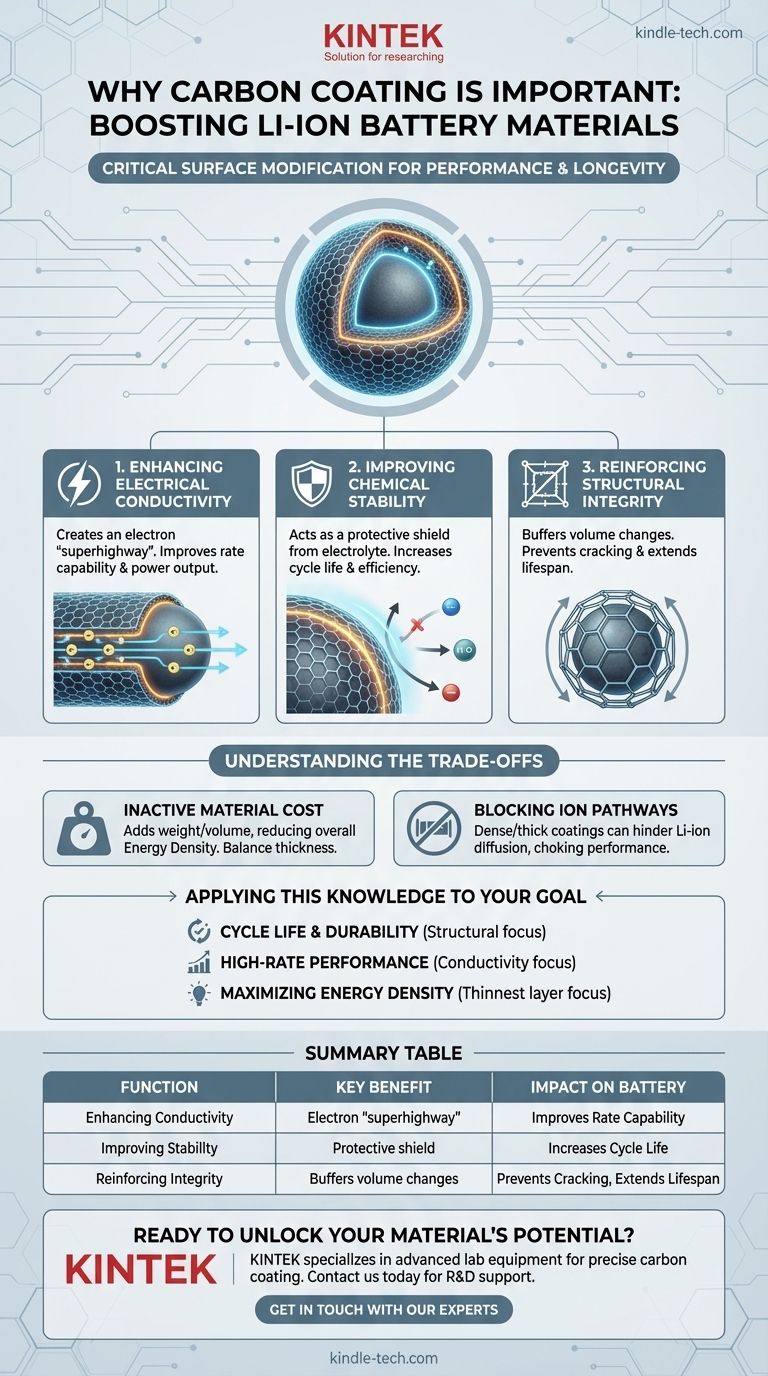
The Three Core Functions of Carbon Coating
To understand why this technique is so essential, we must break down its primary mechanisms of action. Each function addresses a fundamental weakness present in many advanced materials.
1. Enhancing Electrical Conductivity
Many high-capacity battery electrode materials, such as lithium iron phosphate (LFP) or silicon, are unfortunately poor conductors of electrons. This high internal resistance limits how quickly they can be charged and discharged.
The carbon coating creates an incredibly thin, uniform, and highly conductive network around the material particles. This layer acts as a "superhighway" for electrons, ensuring they can move quickly to and from the active material during electrochemical reactions.
This directly translates to better rate capability, meaning the battery can deliver higher power and accept a faster charge without significant performance loss.
2. Improving Chemical Stability
The inside of a battery is a highly reactive environment. The active material on the electrode is in constant contact with a liquid electrolyte, leading to undesirable side reactions.
These reactions consume valuable lithium ions and electrolyte, forming an unstable surface layer known as the Solid Electrolyte Interphase (SEI). This process degrades the battery's capacity and shortens its lifespan.
A carbon coating serves as a physical and chemical shield. It isolates the active material from direct contact with the electrolyte, preventing these parasitic reactions and helping to form a more stable, effective SEI layer. This leads to higher efficiency and a much longer cycle life.
3. Reinforcing Structural Integrity
Many next-generation materials suffer from massive volume changes during charging and discharging. For example, silicon anodes can expand by over 300%, causing the material to crack, pulverize, and lose electrical contact.
The carbon coating acts as an elastic, reinforcing cage around the material. It mechanically buffers the stress of this expansion and contraction, holding the particles together and maintaining the electrode's structural integrity over many cycles.
By preventing this mechanical degradation, the carbon coating ensures the material remains active and connected within the electrode, dramatically extending the battery's operational lifespan.
Understanding the Trade-offs
While immensely beneficial, carbon coating is not a perfect solution and involves critical engineering compromises that must be carefully managed.
The Cost of Inactive Material
The carbon coating itself does not store lithium ions; it is an "inactive" component. Every gram of carbon adds weight and volume to the electrode without contributing to its energy capacity.
Therefore, a key challenge is applying the thinnest possible coating that still provides the necessary conductive and protective benefits. An excessively thick layer will significantly reduce the battery's overall energy density.
The Risk of Blocking Ion Pathways
For the battery to function, lithium ions must be able to move freely from the electrolyte into the active material. The carbon coating must therefore be porous or otherwise structured to allow for this ion diffusion.
A poorly designed coating—one that is too dense or thick—can act as a barrier to lithium ions, effectively choking the battery's performance. This increases resistance and negates the benefits of improved electronic conductivity.
Applying This Knowledge to Your Goal
The optimal carbon coating strategy depends entirely on the primary objective for the final application.
- If your primary focus is maximizing cycle life and durability: The coating's role as a structural reinforcer and chemical shield is paramount, preventing material degradation over thousands of cycles.
- If your primary focus is high-rate performance (power): The enhancement of electronic conductivity is the most critical function, enabling rapid charge and discharge.
- If your primary focus is maximizing energy density: The goal is to engineer the thinnest, most efficient coating possible to minimize inactive mass while still gaining essential stability.
Ultimately, carbon coating is a pivotal engineering tool that unlocks the potential of advanced materials by compensating for their inherent weaknesses.
Summary Table:
| Function | Key Benefit | Impact on Battery Performance |
|---|---|---|
| Enhancing Electrical Conductivity | Creates an electron "superhighway" | Improves rate capability and power output |
| Improving Chemical Stability | Acts as a protective shield from electrolyte | Increases cycle life and efficiency |
| Reinforcing Structural Integrity | Buffers volume changes in active materials | Prevents cracking and extends lifespan |
Ready to unlock the full potential of your battery materials?
At KINTEK, we specialize in advanced lab equipment and consumables for precise carbon coating applications. Whether your goal is maximizing cycle life, achieving high-rate performance, or optimizing energy density, our solutions help you engineer the perfect coating for your specific needs.
Contact us today to discuss how we can support your research and development in creating more durable and efficient energy storage systems.
Visual Guide
Related Products
- Aluminum Foil Current Collector for Lithium Battery
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Platinum Sheet Electrode for Laboratory and Industrial Applications
People Also Ask
- Is chemical Vapour deposition bottom-up approach? Build Materials Atom by Atom
- What is the chemical solution deposition method also known as? Discover the Sol-Gel Process for Thin Films
- What is the manner for depositing extremely controlled thin films? Achieve Atomic-Level Precision with ALD
- What are the advantages of using a Rotary CVD reactor for MWCNTs? Achieve High Consistency and Uniform Growth
- What are the two methods used to deposit thin film components on a substrate? PVD vs. CVD Explained
- What type of power source is used in RF sputtering? High-Frequency AC Solutions for Insulating Materials
- What is the CVD growth process? A Step-by-Step Guide to Chemical Vapor Deposition
- What is the deposition process gas? A Guide to CVD & PVD Gases for Thin Film Deposition


















