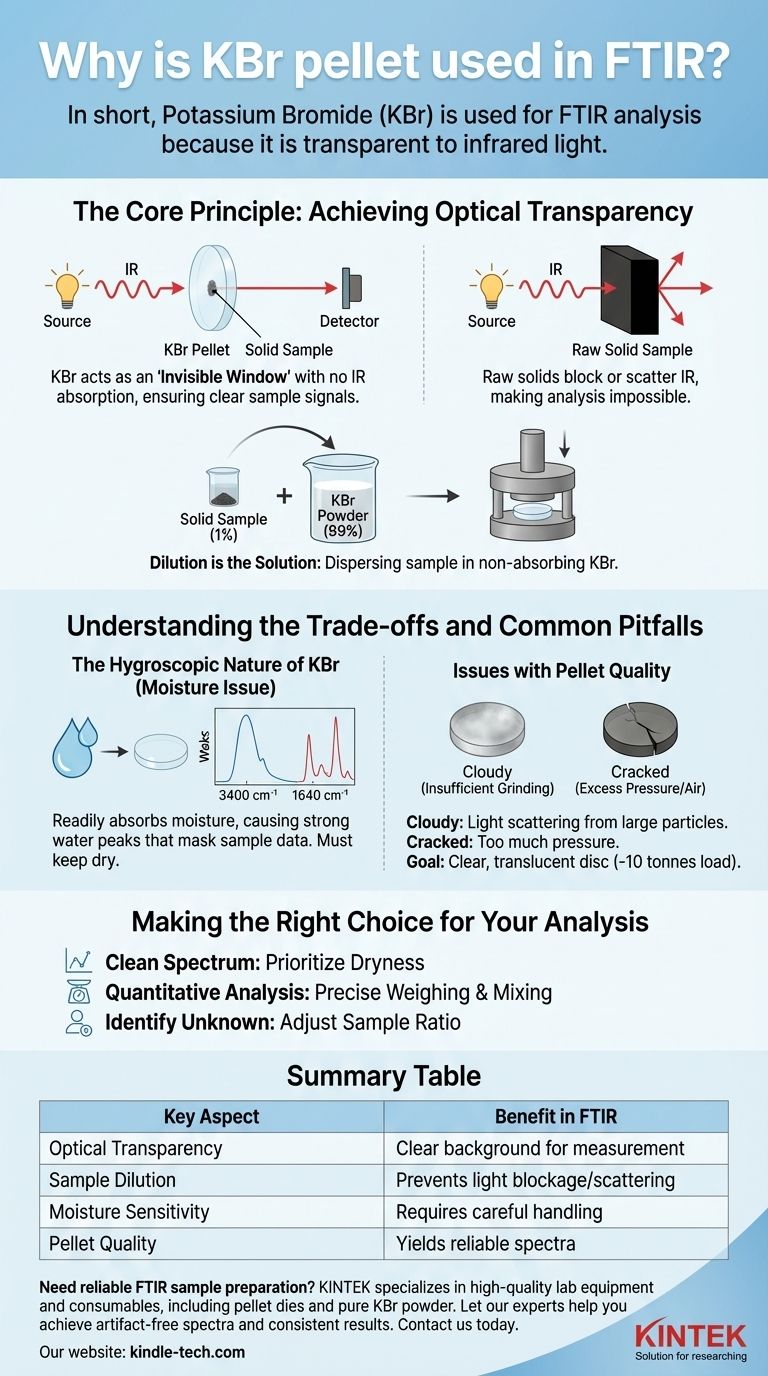
In short, Potassium Bromide (KBr) is used for FTIR analysis because it is transparent to infrared light. This unique property allows it to act as a solid-state "solvent" or matrix for solid samples. By thoroughly mixing a small amount of a solid sample with KBr powder and compressing it into a thin disc, the sample can be held in the path of the instrument's light beam for analysis without the KBr itself absorbing any of the infrared energy and interfering with the measurement.
The core challenge with analyzing solid samples via FTIR is getting the infrared light through the sample without it being completely blocked or scattered. The KBr pellet method solves this by dispersing the sample at a very low concentration within an optically clear, non-absorbing KBr matrix, ensuring a high-quality spectrum.

The Core Principle: Achieving Optical Transparency
Fourier Transform Infrared (FTIR) spectroscopy works by passing an infrared beam through a sample and measuring which frequencies of light are absorbed. For this to work with solids, the sample must be thin and uniform enough for the light to pass through.
KBr's Role as an "Invisible" Window
Potassium Bromide is an ionic salt that does not have molecular bonds that vibrate in the mid-infrared region (typically 4000-400 cm⁻¹). Because it doesn't absorb IR light in this range, it provides a perfectly clear background, ensuring that any absorption peaks detected by the instrument come purely from the sample, not the matrix holding it.
The Problem with Raw Solid Samples
Analyzing a raw, unprepared solid sample is often impossible. A chunk of material is typically too thick and will block the entire IR beam, resulting in no signal. A finely ground powder can be too dense or scatter the light uncontrollably, leading to a distorted and unusable spectrum with a sloping baseline.
Dilution is the Solution
The KBr pellet technique overcomes these issues through dilution. A tiny amount of the solid sample, typically just 1% of the total mass, is mixed with 99% pure KBr.
When this mixture is ground into a fine powder and compressed under high pressure, it forms a solid disc. This process disperses the sample molecules evenly throughout the KBr matrix, creating a "solid solution" that is thin and transparent enough for analysis.
Understanding the Trade-offs and Common Pitfalls
While the KBr pellet method is a gold standard for solid sample analysis, its success depends on careful technique. The most significant challenge is KBr's interaction with water.
The Hygroscopic Nature of KBr
Potassium Bromide is hygroscopic, meaning it readily absorbs moisture (H₂O) from the atmosphere. This is the single most common source of error in this technique.
Water has very strong and broad absorption bands in the infrared spectrum, primarily around 3400 cm⁻¹ (O-H stretching) and 1640 cm⁻¹ (H-O-H bending). If your KBr is "wet," these large water peaks can obscure important peaks from your actual sample.
Mitigating Moisture Contamination
To prevent water contamination, KBr powder should always be stored in a desiccator or dried in an oven before use. The grinding and pressing process should be done as quickly as possible to minimize air exposure. In highly humid environments, using a glovebox or a special vacuum die is recommended.
Issues with Pellet Quality
A high-quality pellet should be thin and translucent, resembling a small piece of glass. Common physical flaws include:
- Cloudy or Opaque Pellets: This is usually caused by insufficient grinding, leading to large KBr particles that scatter light, or insufficient pressure during compression. This results in a noisy, sloping baseline.
- Cracked or Brittle Pellets: This can result from pressing too quickly (trapping air) or using too much pressure, which can sometimes damage the pellet die.
The standard "rule of thumb" is to apply a load of ~10 tonnes using a 13 mm diameter pellet die to achieve a solid, clear disc.
Making the Right Choice for Your Analysis
Properly preparing a KBr pellet is a skill that directly impacts the quality of your spectral data. Your specific goal should guide your focus during preparation.
- If your primary focus is obtaining a clean, artifact-free spectrum: Prioritize using thoroughly dried KBr and minimize its exposure to atmospheric moisture to avoid interfering water peaks.
- If your primary focus is quantitative analysis: Pay meticulous attention to weighing your sample and KBr to maintain a precise ratio (e.g., 1:100) and ensure the powders are ground and mixed until perfectly homogenous.
- If your primary focus is identifying an unknown solid: Begin with the standard 1:100 sample-to-KBr ratio, but be prepared to create a new pellet with more or less sample if your absorption peaks are too weak or completely flattened ("maxed out").
Ultimately, mastering the KBr pellet technique transforms an otherwise opaque solid into an optically perfect sample for definitive infrared analysis.
Summary Table:
| Key Aspect | Benefit in FTIR |
|---|---|
| Optical Transparency | KBr is IR-transparent, providing a clear background for sample measurement. |
| Sample Dilution | Disperses solid samples evenly, preventing light blockage and scattering. |
| Moisture Sensitivity | Hygroscopic nature requires careful handling to avoid water peak interference. |
| Pellet Quality | Proper grinding and pressing (~10 tonnes) yield clear, translucent discs for reliable spectra. |
Need reliable FTIR sample preparation? KINTEK specializes in high-quality lab equipment and consumables, including pellet dies and pure KBr powder, to ensure your solid samples are analyzed with precision. Let our experts help you achieve artifact-free spectra and consistent results. Contact us today to discuss your laboratory needs!
Visual Guide
Related Products
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Laboratory Hydraulic Press Lab Pellet Press Machine for Glove Box
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
People Also Ask
- What is an example of a hydraulic press? Discover the Power of Laboratory Sample Preparation
- How does pressure affect hydraulic system? Mastering Force, Efficiency, and Heat
- What is a hydraulic press for sample preparation? Create Consistent Pellets for Reliable Analysis
- What is the purpose of KBr pellets? Unlock Clear FTIR Analysis of Solid Samples
- Why do we use KBr in FTIR? The Key to Clear, Accurate Solid Sample Analysis



















