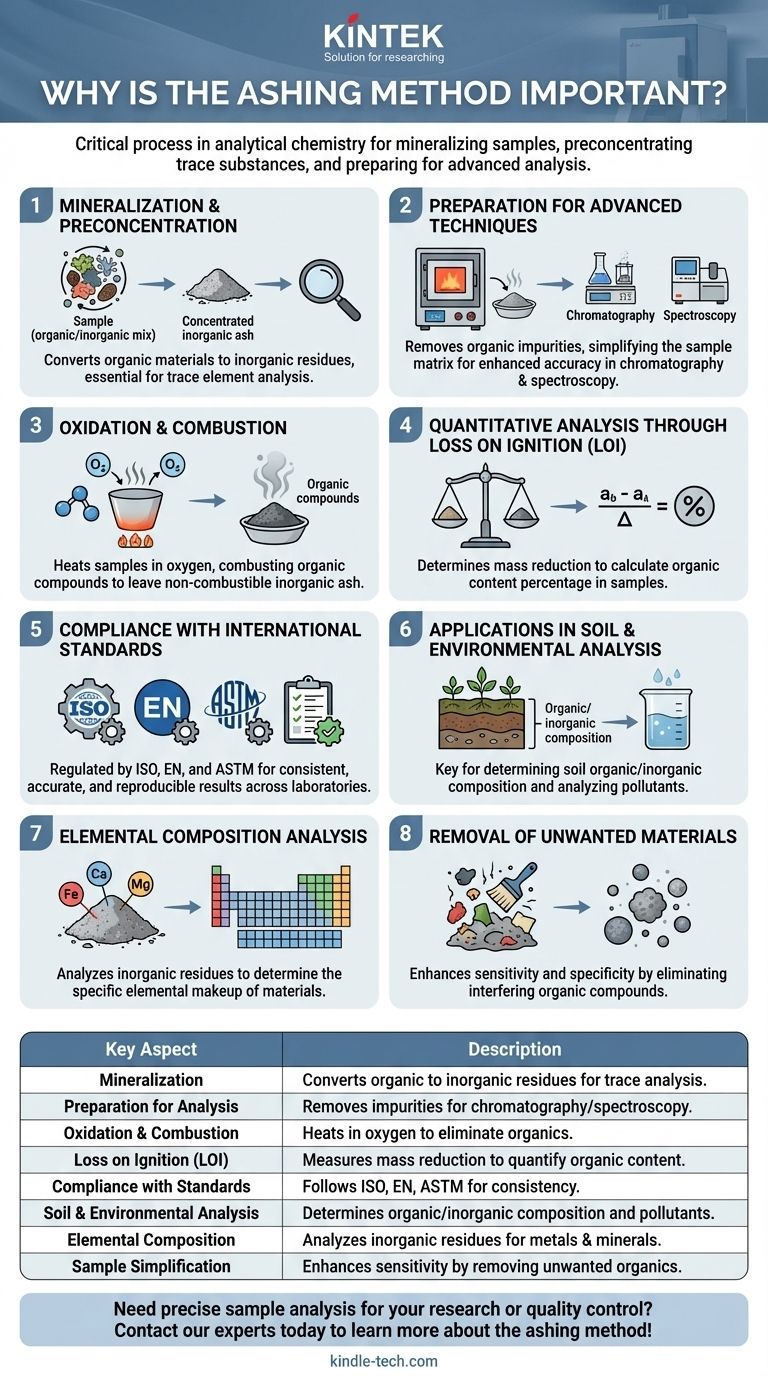
The ashing method is a critical process in analytical chemistry, primarily used for the mineralization of samples to preconcentrate trace substances for subsequent chemical or optical analysis. By heating samples in the presence of oxygen, organic compounds are oxidized and combusted, leaving behind inorganic, non-combustible residues. This method is essential for determining the elemental composition of materials, analyzing soil organic content, and preparing samples for techniques like chromatography or spectroscopy. Governed by international standards, ashing ensures accuracy and consistency in analytical results, making it indispensable in research, quality control, and environmental analysis.

Key Points Explained:
-
Mineralization and Preconcentration of Trace Substances:
- Ashing is a process that converts organic materials into inorganic residues by combustion, effectively mineralizing the sample.
- This step is crucial for preconcentrating trace elements or substances, making them easier to detect and analyze in subsequent tests.
- For example, in soil analysis, ashing helps isolate inorganic components, allowing for accurate measurement of organic content.
-
Preparation for Advanced Analytical Techniques:
- The ashing process prepares samples for techniques like chromatography and spectroscopy by removing organic impurities.
- By leaving behind only inorganic residues, ashing simplifies the sample matrix, enhancing the accuracy and reliability of analytical results.
- This is particularly important in fields like environmental science, where precise measurement of trace elements is required.
-
Oxidation and Combustion of Organic Compounds:
- During ashing, samples are heated in the presence of oxygen, causing organic compounds to oxidize and combust.
- This step eliminates unwanted organic materials, leaving behind non-combustible inorganic ash.
- The process is governed by strict protocols to ensure complete combustion and consistent results.
-
Quantitative Analysis Through Loss on Ignition (LOI):
- Ashing is often used to determine the mass reduction of a sample, known as Loss on Ignition (LOI).
- By weighing the sample before and after ashing, the proportion of organic material can be calculated.
- This method is widely used in soil analysis to assess organic content and in industries like food and pharmaceuticals to evaluate purity.
-
Compliance with International Standards:
- The ashing process is regulated by international standards such as ISO, EN, and ASTM.
- These standards ensure that the method is performed consistently and accurately across different laboratories and industries.
- Compliance with these standards is essential for maintaining the credibility and reproducibility of analytical results.
-
Applications in Soil and Environmental Analysis:
- Ashing is a key technique in soil analysis, where it helps determine the organic and inorganic composition of soil samples.
- By comparing the mass before and after ashing, researchers can quantify the organic matter content, which is vital for agricultural and environmental studies.
- This method is also used in environmental monitoring to analyze pollutants and trace elements in various samples.
-
Elemental Composition Analysis:
- The residual ash left after ashing contains inorganic compounds that can be analyzed to determine the elemental composition of the original sample.
- This is particularly useful in industries like metallurgy, where the presence of specific metals or minerals needs to be quantified.
- Ashing ensures that only the relevant inorganic components are analyzed, reducing interference from organic materials.
-
Removal of Unwanted Materials:
- By eliminating organic compounds, ashing simplifies the sample, making it easier to analyze the remaining inorganic residues.
- This is especially important in complex samples where organic and inorganic components are intermixed.
- The process enhances the sensitivity and specificity of subsequent analytical techniques.
In summary, the ashing method is a foundational technique in analytical chemistry, enabling the accurate and reliable analysis of trace substances, elemental composition, and organic content. Its importance lies in its ability to simplify complex samples, comply with international standards, and provide precise results for a wide range of applications.
Summary Table:
| Key Aspect | Description |
|---|---|
| Mineralization | Converts organic materials into inorganic residues for trace substance analysis. |
| Preparation for Analysis | Removes organic impurities, simplifying samples for chromatography/spectroscopy. |
| Oxidation & Combustion | Heats samples in oxygen to eliminate organic compounds, leaving inorganic ash. |
| Loss on Ignition (LOI) | Measures mass reduction to quantify organic content in samples. |
| Compliance with Standards | Follows ISO, EN, and ASTM standards for consistent and accurate results. |
| Soil & Environmental Analysis | Determines organic/inorganic composition in soil and pollutant analysis. |
| Elemental Composition | Analyzes inorganic residues to quantify metals and minerals in samples. |
| Sample Simplification | Enhances sensitivity and specificity by removing unwanted organic materials. |
Need precise sample analysis for your research or quality control? Contact our experts today to learn more about the ashing method!
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the use of muffle furnace in food industry? Essential for Accurate Food Ash Analysis
- For what purpose is a programmed temperature heat treatment furnace used when testing MPCF/Al composites? Space Testing
- How do you cool a muffle furnace? Safeguard your equipment and samples from thermal shock.
- Why do we need to use properly some of the laboratory apparatus in the laboratory? The Foundation of Safe and Accurate Science
- What are the risks of using a muffle furnace? Mitigate Thermal, Material, and Operational Hazards



















