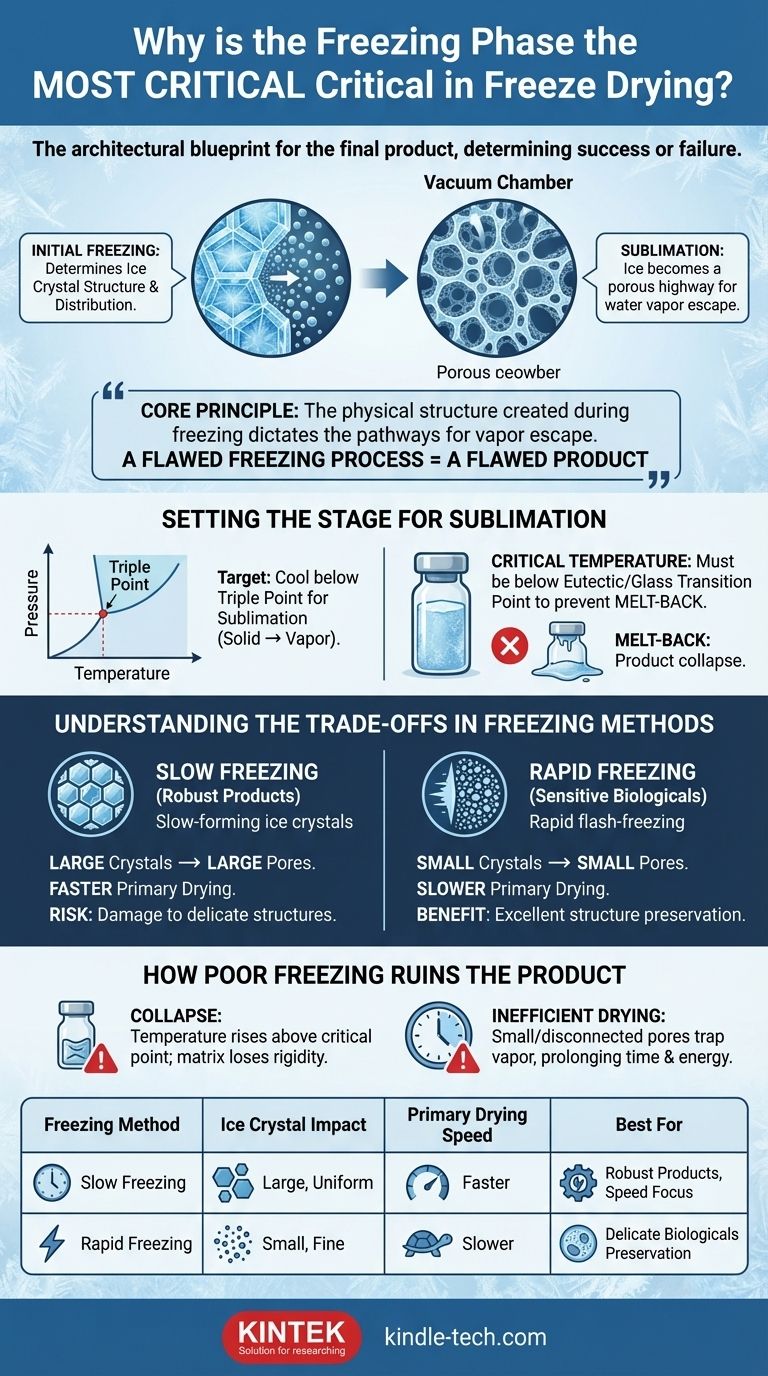
In freeze-drying, the freezing phase is the most critical step because it acts as the architectural blueprint for the final product. This initial stage determines the ice crystal structure, which directly controls the rate and success of the subsequent drying phases. A mistake made here cannot be corrected later in the process.
The core principle to understand is that the physical structure created during freezing—specifically the size and distribution of ice crystals—dictates the pathways through which water vapor will escape during drying. A flawed freezing process results in a flawed product.

Why Freezing Governs the Entire Process
The primary goal of freeze-drying, or lyophilization, is to remove water without damaging the product's structure. This is achieved by converting solid ice directly into vapor, a process called sublimation. The freezing stage makes this possible.
Setting the Stage for Sublimation
For sublimation to occur, the material must be cooled below its triple point, the unique temperature and pressure at which a substance can exist as a solid, liquid, and gas simultaneously.
By completely freezing the product, you ensure all water is locked into a solid state. This prevents the material from melting under vacuum, which would destroy its delicate structure.
The Critical Role of Ice Crystal Structure
As water freezes, it forms ice crystals. These crystals push aside and concentrate the other components of the product.
When sublimation begins, these ice crystals vacate, leaving behind a network of pores or channels in their place. The size and shape of these pores are a direct copy of the ice crystals that formed them. This porous network is the highway system that water vapor uses to escape the product.
Achieving the Critical Temperature
Every product has a critical temperature, often called the eutectic point or glass transition temperature. This is the minimum temperature at which the product is guaranteed to be completely solid.
Failing to cool the product below this point is the most common cause of failure. Any unfrozen liquid will boil instead of sublimating, causing the product's structure to collapse in on itself, a phenomenon known as melt-back.
Understanding the Trade-offs in Freezing Methods
The rate at which you freeze the product directly influences ice crystal size, presenting a critical trade-off between drying speed and structural preservation.
The Impact of Slow Freezing
Slowing the freezing process allows larger, more uniform ice crystals to form.
This creates large, interconnected pores, which allows water vapor to escape very efficiently. The result is a much faster primary drying phase. However, these large crystals can damage or destroy delicate structures like cell walls.
The Impact of Rapid Freezing
Freezing the product very quickly, such as by flash-freezing in liquid nitrogen, traps the water in place, forming very small ice crystals.
This method is excellent for preserving sensitive biological structures. The downside is that the resulting small pores create more resistance for the escaping water vapor, significantly slowing down the drying process.
How Poor Freezing Ruins the Final Product
The consequences of an improper freezing protocol are irreversible and typically catastrophic for the batch.
The Risk of Collapse
If the product temperature rises above its critical eutectic point during primary drying, the frozen matrix will lose its rigidity and collapse. This results in a shrunken, sticky, and unusable product that has lost its intended structure.
Inefficient Drying Cycles
Even if a full collapse is avoided, a poor freezing structure can drastically prolong the drying time. Small or poorly connected pores trap water vapor, making it difficult to reach the desired final moisture level and wasting significant time and energy.
Making the Right Choice for Your Goal
The optimal freezing strategy depends entirely on the nature of your product and your desired outcome.
- If your primary focus is preserving delicate biologicals (e.g., proteins, bacteria): Opt for rapid freezing to create small ice crystals that protect cellular integrity, even if it extends drying time.
- If your primary focus is processing speed for a robust product (e.g., simple chemical solutions, some foods): A slower, controlled freeze will generate larger crystals, significantly shortening the drying cycle.
- If your primary focus is a complex formulation with variable components: Consider an annealing step, where the product is frozen, slightly warmed, and then re-frozen to encourage uniform crystal growth and improve stability.
Ultimately, mastering the freezing phase is the single most important factor in achieving a successful, high-quality freeze-dried product.
Summary Table:
| Freezing Method | Impact on Ice Crystals | Primary Drying Speed | Best For |
|---|---|---|---|
| Slow Freezing | Large, uniform crystals | Faster | Robust products, speed focus |
| Rapid Freezing | Small, fine crystals | Slower | Delicate biologicals, structure preservation |
Achieve perfect lyophilization results with KINTEK.
The freezing phase is the foundation of a successful freeze-drying cycle. Ensuring your product is frozen correctly requires precise and reliable equipment. KINTEK specializes in high-quality lab equipment and consumables for all your laboratory needs, including advanced freeze-drying solutions.
Our expertise can help you select the right equipment to master the critical freezing step, ensuring optimal ice crystal structure, efficient drying cycles, and a perfect final product every time.
Contact our experts today to discuss how we can support your specific freeze-drying challenges and enhance your lab's efficiency.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
People Also Ask
- Why is a freeze dryer preferred for drying nickel nanoparticle precursors? Prevent Hard Agglomeration Now
- What are the advantages of using freeze drying for phase change materials with biopolymer shells? Optimize Stability
- Why is a freeze dryer preferred over thermal drying for Fe-ZTA cermets? Ensure Pure, Homogeneous Slurry Processing
- What is the purpose of an evaporator? The Key Component That Creates Cooling
- What is the function of a freeze dryer in the ice-templating process? Preserving Aligned Pore Scaffolds for LAGP



















