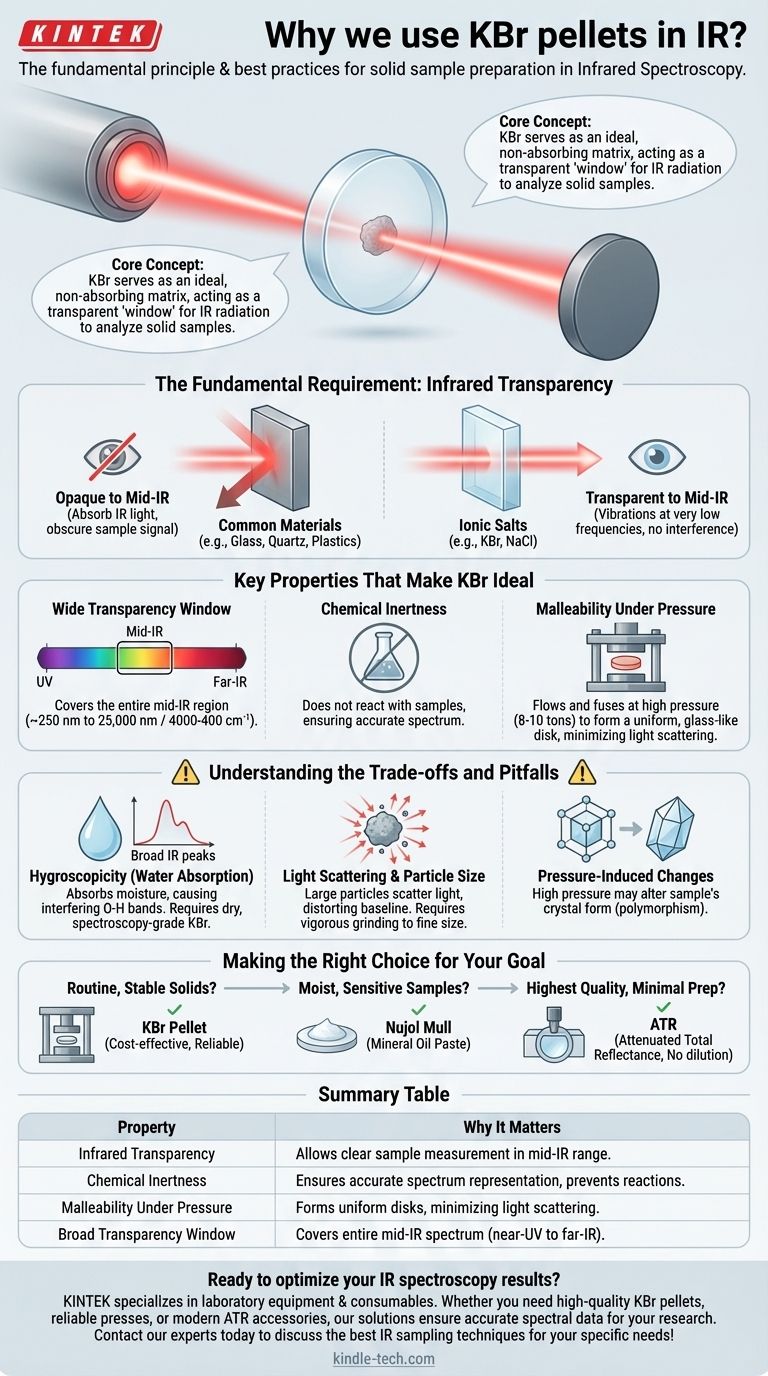
At its core, we use Potassium Bromide (KBr) to prepare solid samples for Infrared (IR) spectroscopy because it is transparent to infrared radiation and can be pressed into a thin, solid disk. This KBr disk acts as a window, holding the sample in the path of the IR beam without producing any interfering signals of its own.
The fundamental challenge in IR spectroscopy of solids is getting the light through the sample without the sample holder itself absorbing that light. KBr serves as an ideal, non-absorbing matrix that allows for the clear measurement of the sample's unique molecular vibrations.

The Fundamental Requirement: Infrared Transparency
To obtain a useful IR spectrum, the material holding your sample must not absorb IR radiation in the same region as your compound of interest. This is the single most important criterion.
Why Most Materials Are Unsuitable
Common materials like glass, quartz, or plastics are opaque to most of the mid-infrared spectrum (4000-400 cm⁻¹). Their own covalent bonds vibrate and absorb IR light, which would completely obscure the signal from your sample.
The Advantage of Ionic Salts
Alkali halides, a class of ionic salts that includes Potassium Bromide (KBr) and Sodium Chloride (NaCl), are different. The vibrations of their strong ionic crystal lattices occur at very low frequencies, far below the typical mid-IR range.
This property makes them effectively transparent across the spectral region where the vibrations of organic and inorganic molecules are found.
Key Properties That Make KBr Ideal
While other salts like NaCl are also IR transparent, KBr is the most common choice for making sample pellets due to a combination of beneficial properties.
Wide Transparency Window
KBr is transparent over a very broad range, from the near-UV (~250 nm) all the way to the far-IR (~25,000 nm or 400 cm⁻¹). This covers the entire mid-IR region of interest for most chemical analyses.
Chemical Inertness
For the vast majority of analyses, KBr is chemically inert. It does not react with the sample, ensuring the spectrum you measure is that of your unaltered compound.
Malleability Under Pressure
KBr is a relatively soft, plastic-like salt. When subjected to high pressure (typically 8-10 tons), the KBr powder flows and fuses together, forming a semi-transparent, glass-like disk. This process encapsulates the finely ground sample particles in a uniform matrix.
This uniform matrix is crucial for minimizing the scattering of IR light, which would otherwise distort the spectrum's baseline.
Understanding the Trade-offs and Pitfalls
While the KBr pellet technique is a classic and effective method, it is not without significant challenges. Acknowledging these is critical for obtaining high-quality data.
The Problem with Water (Hygroscopicity)
This is the most common pitfall. KBr is hygroscopic, meaning it readily absorbs moisture from the atmosphere. This water will appear in your spectrum as very broad absorption bands around 3400 cm⁻¹ (O-H stretching) and 1640 cm⁻¹ (H-O-H bending).
These water peaks can easily obscure important sample signals, such as N-H or O-H stretches. Always use spectroscopy-grade KBr that has been thoroughly dried in an oven and stored in a desiccator.
Light Scattering and Particle Size
If the sample is not ground into particles smaller than the wavelength of the IR light, significant light scattering can occur. This leads to a sloping baseline and distorted peak shapes, an artifact known as the Christiansen effect.
Proper sample preparation requires grinding the sample and KBr together vigorously in an agate mortar and pestle to ensure a fine, homogenous mixture.
Pressure-Induced Changes
The high pressure used to form the pellet can sometimes induce changes in the crystal form of the sample (polymorphism). This means the spectrum you obtain may not be perfectly representative of the sample in its original, un-pressed state.
Making the Right Choice for Your Goal
Modern techniques have provided alternatives to KBr pellets. Your choice of sampling method should be guided by your specific sample and analytical goal.
- If your primary focus is routine analysis of stable, non-moisture-sensitive solids: The KBr pellet method remains a cost-effective and reliable technique when performed carefully.
- If your sample is moist, difficult to grind, or sensitive to pressure: Consider a Nujol mull, where the solid is ground into a mineral oil paste that is then spread between two salt plates.
- If you need the highest quality data with minimal sample preparation: Attenuated Total Reflectance (ATR) is the modern gold standard for most solid and liquid samples, requiring no sample dilution and removing nearly all the issues associated with pellets.
Understanding the principles behind your chosen sampling technique is the first step toward acquiring a meaningful and accurate spectrum.
Summary Table:
| Property | Why It Matters for IR Spectroscopy |
|---|---|
| Infrared Transparency | KBr doesn't absorb IR light in the mid-IR range (4000-400 cm⁻¹), allowing clear sample measurement |
| Chemical Inertness | Prevents reactions with your sample, ensuring accurate spectrum representation |
| Malleability Under Pressure | Forms uniform, transparent disks that minimize light scattering when pressed |
| Broad Transparency Window | Covers entire mid-IR spectrum from near-UV to far-IR regions |
Ready to optimize your IR spectroscopy results? KINTEK specializes in laboratory equipment and consumables for precise sample preparation. Whether you need high-quality KBr pellets, reliable presses, or modern ATR accessories, our solutions ensure accurate spectral data for your research. Contact our experts today to discuss the best IR sampling techniques for your specific laboratory needs!
Visual Guide
Related Products
- kbr pellet press 2t
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
People Also Ask
- What is the function of a hydraulic forging press? Shape Metal with Unmatched Force and Control
- What are the advantages of using a laboratory manual hydraulic pellet press for FTIR? Enhance Your Spectral Data
- What are the features of a hydraulic press? Unlock Immense Force with Simple, Reliable Design
- What is the application of a laboratory hydraulic press in PHA testing? Perfecting Biopolymer Material Analysis
- What key indicators does a laboratory pressure testing machine measure? Essential T91 Alloy Steel Weld Testing
- What is hydraulic press forging? Achieve Precise Control and Superior Strength for Large Metal Parts
- What's the difference between cold press and regular press? Choosing Between Quality and Efficiency
- What is a 100 ton press used for? A Guide to Industrial Bending, Forming, and Assembly



















