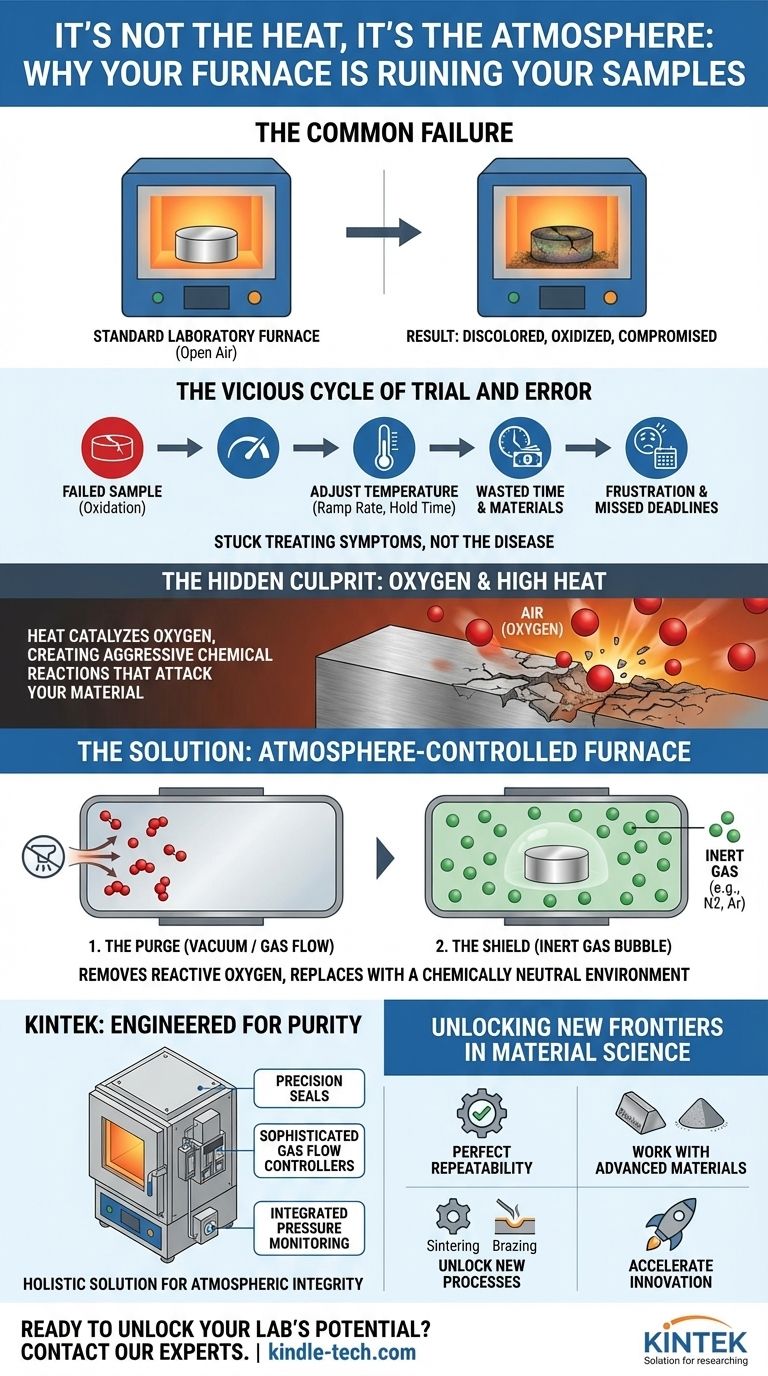
You’ve spent days preparing a pristine metal component or a carefully synthesized powder. Every variable is accounted for. You place it in your standard laboratory furnace, set the precise temperature profile, and wait. Hours later, you open the door, expecting perfection. Instead, you find a discolored, oxidized, and fundamentally compromised version of what you put in. The surface is tarnished, the properties are wrong, and the sample is useless.
That sinking feeling is a familiar one in labs and R&D departments everywhere.
The Vicious Cycle of Trial and Error
When a high-temperature process fails, our instincts tell us to blame the most obvious variable: the heat. We ask ourselves:
- "Was the temperature ramp rate too fast?"
- "Did I hold it at the peak temperature for too long?"
- "Is this batch of raw material somehow defective?"
So begins a frustrating and expensive cycle of troubleshooting. You adjust the temperature profiles, run the process again, and get another inconsistent result. Each failed attempt not only consumes valuable materials and energy but also erodes something far more critical: time. Project deadlines slip, R&D milestones are missed, and confidence in your process evaporates. The commercial consequences are stark—delayed product launches, unreliable quality control, and an inability to work with next-generation, high-performance materials.
You're stuck treating the symptoms, because you haven't yet identified the real disease.
The Hidden Culprit: Why Air Becomes the Enemy
The fundamental misunderstanding is that heat is the only active agent in your furnace. The real problem is what the heat does to the seemingly harmless air filling the chamber.
At room temperature, the oxygen in the air is relatively benign. But as you apply intense heat, you transform it into a highly aggressive chemical reactant. The heat acts as a catalyst, dramatically accelerating the process of oxidation. This unwanted chemical reaction is what attacks the surface of your material, creating the scale, tarnish, and brittleness that ruins your work.
This is precisely why your previous attempts failed. Tweaking the temperature is like trying to stay dry in a rainstorm by changing your walking speed—as long as you’re in the rain, you’re going to get wet. As long as your material is surrounded by oxygen at high temperatures, it is going to oxidize. The only way to guarantee a perfect outcome is to get rid of the oxygen altogether.
Fighting Chemistry with Chemistry: The Role of a Controlled Atmosphere
To solve a problem of chemistry, you need a tool designed for chemistry. You must remove the reactive element (oxygen) and replace it with an environment that is chemically neutral, even at extreme temperatures.
This is the entire principle behind an atmosphere-controlled furnace. It’s not just a box that gets hot; it’s a precision environmental chamber engineered to solve this exact problem. It works in two critical stages:
- The Purge: First, it uses a vacuum or a flow of gas to completely remove the reactive, oxygen-rich air from its sealed chamber.
- The Shield: Then, it introduces an inert gas, like Nitrogen or Argon, creating a stable, protective "bubble" around your material. This gas shield will not react with your sample, no matter how high the temperature gets.
KINTEK: Furnaces Engineered for Purity
A truly effective solution requires more than just a gas inlet. It requires a system built from the ground up for atmospheric integrity. KINTEK's atmosphere-controlled furnaces are designed not as an afterthought, but as a holistic solution to the problem of oxidation. Key features like precision-machined seals, sophisticated gas flow controllers, and integrated pressure monitoring systems work in concert to ensure that the protective atmosphere you set is the atmosphere your sample actually experiences—from the beginning of the cycle to the very end. This isn't just a better furnace; it's the right tool for the job.
Beyond Prevention: Unlocking New Frontiers in Material Science
Once you eliminate the constant, nagging problem of oxidation, you do more than just prevent failures. You open up a whole new realm of possibilities for your research and production.
With a reliable, controlled atmosphere, you can finally:
- Achieve Perfect Repeatability: Produce identical, high-quality results every single time, making your data more reliable and your quality control ironclad.
- Work with Advanced Materials: Confidently process highly sensitive materials like titanium alloys, powdered metals, and technical ceramics that are impossible to handle in open air.
- Unlock New Processes: Master advanced thermal processes like sintering, brazing, and bright annealing that are fundamentally dependent on a controlled atmosphere.
- Accelerate Innovation: Stop wasting weeks troubleshooting and start dedicating that time to developing the next generation of materials and products.
Your challenge isn't just about preventing a discolored sample; it's about achieving breakthrough results, faster and more reliably than ever before. If you're ready to move past the limitations of conventional heating and unlock your lab's true potential, our team is here to help you design the right process. Contact Our Experts to discuss your unique material challenges.
Visual Guide
Related Products
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
Related Articles
- Why Your Brazed Joints Keep Failing: The Invisible Saboteur in Your Furnace
- The Benefits of Controlled Atmosphere Furnaces for Sintering and Annealing Processes
- Muffle Furnace: Unraveling the Secrets of Uniform Heating and Controlled Atmosphere
- Exploring the Using a Chamber Furnace for Industrial and Laboratory Applications
- Controlled Atmosphere Furnace: Comprehensive Guide to Advanced Heat Treatment




















