You’ve done everything by the book. The parts were meticulously cleaned, the filler metal was correct, and the temperature profile was executed perfectly. Yet, when you pull the parts from the furnace, the inspection reveals a disaster: weak, discolored joints that are immediately rejected. It’s a frustrating, head-scratching scenario that plays out in labs and production facilities far too often. Weeks can be lost trying to pinpoint a cause that remains stubbornly elusive.
Chasing Ghosts: Why Common Fixes Don't Work
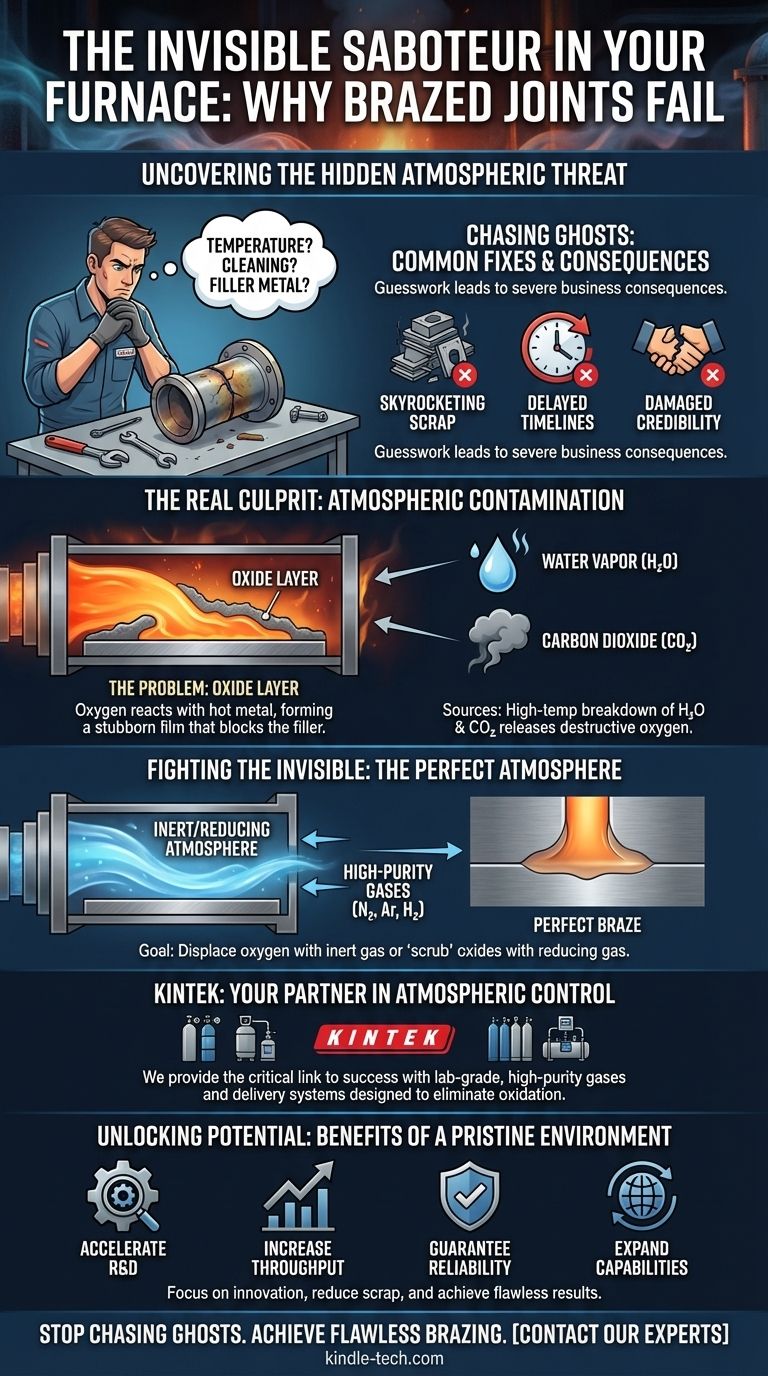
When faced with a failed braze, the troubleshooting checklist is predictable. Did we get the temperature right? Let's recalibrate the controller. Were the parts truly clean? Let's try a different cleaning agent. Is it the filler metal? Let's order a new batch.
Each attempt is a shot in the dark, a process of elimination that consumes valuable time and expensive materials. While these factors are important, focusing on them alone is like trying to fix a flickering light by changing the lightbulb when the real problem is faulty wiring in the wall. These "solutions" often fail because they don't address the true root cause.
The business consequences are severe. This guesswork leads to:
- Skyrocketing Scrap Rates: Expensive components and alloys are wasted.
- Delayed Timelines: Projects are pushed back, and production schedules are thrown into chaos.
- Damaged Credibility: Shipping a product with a potentially weak joint isn't just a quality issue; it's a major liability, especially in critical applications like aerospace or medical devices.
The Real Culprit: It’s Not the Heat, It’s the Atmosphere
The turning point in solving this problem is to stop looking at the visible components—the parts and the filler—and start focusing on the invisible environment they are heated in: the furnace atmosphere.
The Science of a Failed Joint: Meet the Oxide Layer
At high brazing temperatures, metals are incredibly reactive. If even a few molecules of oxygen are present, they will instantly bond with the hot metal to form a microscopic, stubborn film of oxide. Think of it as a near-instantaneous layer of rust.
This oxide layer acts as a physical barrier. No matter how hot the furnace gets, the molten filler metal cannot "wet" or bond with the parent material because this oxide wall is in the way. The result is a weak joint, or no joint at all.
The Hidden Sources of Oxygen
The real challenge is that oxygen is a master of disguise. It doesn't just come from an obvious air leak. The most common saboteurs are:
- Water Vapor (H₂O): Often the biggest offender. At high temperatures, water molecules split apart, releasing a steady stream of destructive oxygen directly onto your parts. The "dew point" of your atmosphere gas is a direct measure of this hidden threat.
- Carbon Dioxide (CO₂): Like water vapor, CO₂ can also break down under heat and release oxygen, creating an oxidizing environment that ruins the braze.
This is why your previous fixes failed. You could have the cleanest parts in the world, but if they are heated in an atmosphere contaminated with trace amounts of water vapor, they will re-oxidize inside the furnace, and the braze will fail.
Fighting an Invisible Enemy: The Role of a Perfect Atmosphere
To achieve a consistently perfect braze, you must shift your goal from "cleaning the parts" to "protecting the parts." The only way to do this is by creating and maintaining an atmosphere inside the furnace that is fundamentally incapable of forming oxides.
This requires a meticulously controlled environment, created by flushing the furnace with a gas that displaces every trace of oxygen and its sources. This could be:
- An Inert Atmosphere (like high-purity Nitrogen or Argon): These gases act as a protective shield, creating a neutral space where oxidation simply cannot occur.
- A Reducing Atmosphere (containing Hydrogen): This type of atmosphere goes a step further. It not only displaces oxygen but also actively "scrubs" away any light, pre-existing oxides from the parts by reacting with them.
Creating and sustaining this perfect atmosphere isn't a matter of chance; it requires the right tools for the job.
KINTEK: Your Partner in Atmospheric Control
To achieve this level of atmospheric purity, you need more than just a standard tank of gas. You need a reliable source of high-purity gases and consumables specifically designed for these demanding applications.
This is precisely where KINTEK provides the critical link to success. Our solutions are engineered based on a deep understanding of brazing chemistry. We provide the lab-grade, high-purity gases (like Argon, Nitrogen, and Hydrogen blends) and the delivery systems needed to eliminate the threat of oxygen, water vapor, and CO₂. KINTEK's offerings are not just products; they are the embodiment of the solution, designed to directly address the root cause of oxidation and ensure your furnace environment is pristine, every single time.
From Troubleshooting to Innovation: What’s Possible with Perfect Brazing
Once you stop fighting inconsistent results, you unlock a new level of potential. Instead of wasting resources on troubleshooting, your team can:
- Accelerate R&D: Confidently develop products using more advanced, sensitive materials and complex joint designs.
- Increase Throughput: Dramatically reduce scrap rates from double-digits to near-zero, improving efficiency and profitability.
- Guarantee Reliability: Manufacture mission-critical components with the certainty that every single joint is as strong and reliable as the last.
- Expand Capabilities: Take on more challenging projects that were previously deemed too risky or difficult to braze consistently.
Solving your brazing problem is about more than just fixing a failed joint. It's about transforming a source of frustration into a competitive advantage. Your team's expertise can finally be focused on innovation, not on repetitive troubleshooting. If you're ready to stop chasing ghosts in your furnace and achieve flawless results, our experts are here to help. Let's talk about the specific challenges you face and build a robust process for your unique application. Contact Our Experts.
Visual Guide
Related Products
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vertical Laboratory Tube Furnace
Related Articles
- How Controlled Atmosphere Furnaces Improve Quality and Consistency in Heat Treatment
- Muffle Furnace: Unraveling the Secrets of Uniform Heating and Controlled Atmosphere
- Exploring Tungsten Vacuum Furnaces: Operation, Applications, and Advantages
- Atmosphere Furnaces: Comprehensive Guide to Controlled Heat Treatment
- Hydrogen Atmosphere Furnaces: Applications, Safety, and Maintenance




















