The Myth of the Monolith
In experimental science, we often trust our tools more than we trust our theories. We assume the container is passive. We assume the vessel is silent.
But in electrochemistry, no vessel is truly silent.
We frequently encounter the term "all-quartz electrolytic cell." It sounds absolute. It suggests a singular, uninterrupted material ensuring total purity.
This is a misnomer.
If a cell were truly 100% quartz—body, lid, and threads—it would likely fail. It would be brittle, impossible to seal tight, and prone to shattering under torque.
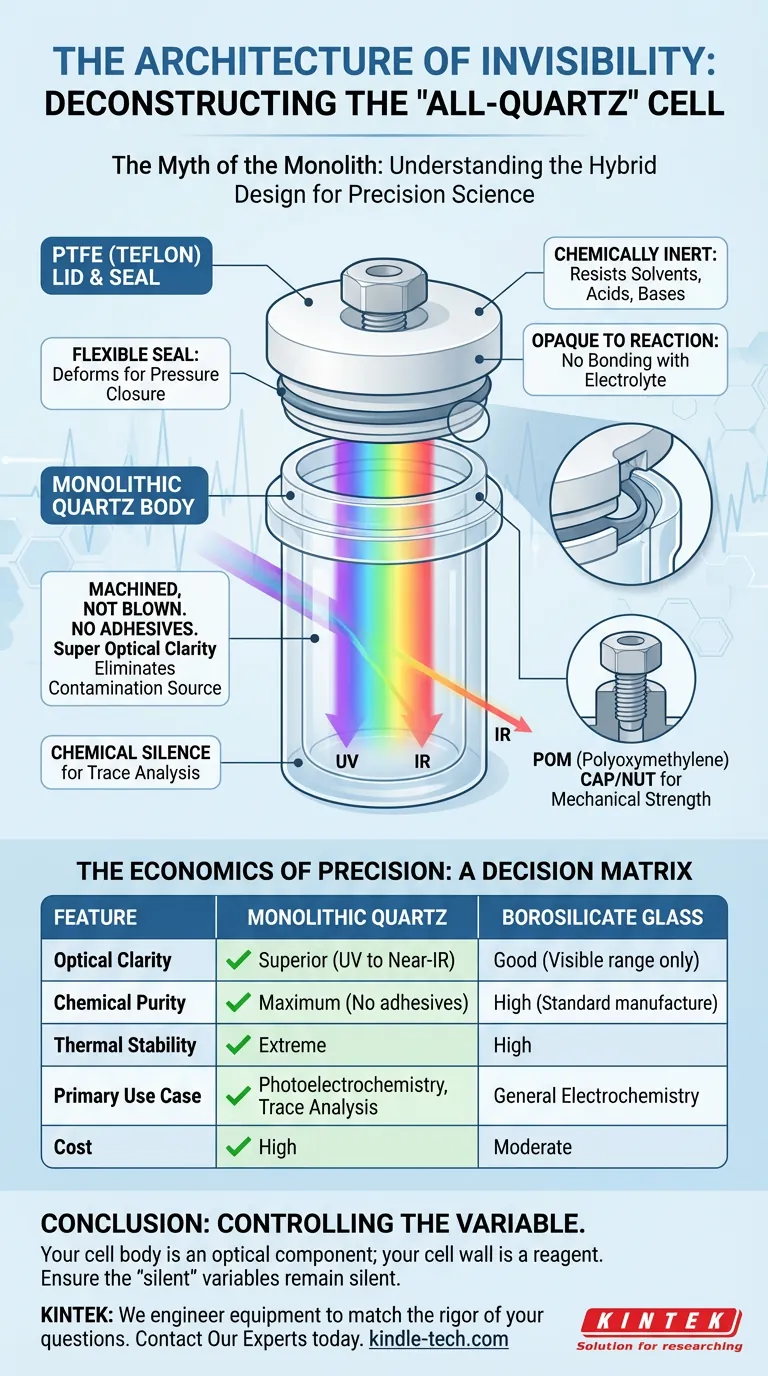
The "all-quartz" cell is actually a sophisticated marriage of two distinct materials: a monolithic quartz body and a high-performance polymer lid. Understanding this interface is not just about knowing parts; it is about understanding where your data comes from.
The Body: Machined for Silence
The heart of the cell is the body. In high-end applications, this is not blown glass; it is machined from a monolithic block of high-purity quartz.
Why go to this trouble? Why avoid the cheaper, easier method of gluing components together?
Because in trace analysis, adhesive is noise.
Glues leach. Adhesives degrade. In the presence of aggressive electrolytes, a bonded joint is a potential contamination source that introduces "ghost" peaks to your data. A monolithic quartz body removes the variable of adhesive failure entirely.
The Optical Imperative
Beyond chemical silence, quartz offers the engineering equivalent of invisibility.
Standard glass blocks ultraviolet light. For a photoelectrochemist, this is catastrophic. It is like trying to study the sun while wearing a blindfold.
Quartz provides exceptional optical transparency from the deep UV to the near-infrared spectrum. It allows the light to interact with the sample without the container absorbing the energy.
The Lid: The Necessary Compromise
While the body handles the light and the chemistry, the lid handles the mechanics. This is where PTFE (Polytetrafluoroethylene) enters the equation.
You likely know it as Teflon.
We do not use quartz for the lid because quartz does not flex. A seal requires a material that can deform slightly under pressure to close gaps. PTFE is the perfect partner for quartz because:
- It is chemically inert: It survives solvents, acids, and bases that destroy lesser polymers.
- It is opaque to reaction: It refuses to bond with the electrolyte.
In some designs, you may also find POM (Polyoxymethylene) used for external screw nuts or caps. These provide the mechanical strength to hold the seal, while the PTFE ensures the chemistry remains pure.
The Economics of Precision
Why doesn't everyone use quartz?
Because precision is expensive.
In the psychology of laboratory procurement, we face a constant trade-off between the "Good Enough" and the "Perfect."
Borosilicate glass is the "Good Enough." It is the workhorse. It offers decent chemical resistance and is cost-effective. For standard electrochemistry where UV transmission is irrelevant, it is the rational choice.
But "rational" changes depending on your goal. If you save money on glass but lose weeks of time wondering why your UV-Vis spectrum is cut off, you haven't saved anything.
The Decision Matrix
Here is how to navigate the trade-off without over-engineering or under-specifying:
| Feature | Monolithic Quartz | Borosilicate Glass |
|---|---|---|
| Optical Clarity | Superior (UV to Near-IR) | Good (Visible range only) |
| Chemical Purity | Maximum (No adhesives) | High (Standard manufacture) |
| Thermal Stability | Extreme | High |
| Primary Use Case | Photoelectrochemistry, Trace Analysis | General Electrochemistry |
| Cost | High | Moderate |
Conclusion: Controlling the Variable
Science is the art of controlling variables.
If your work involves photoelectrochemistry, the cell body is not just a container; it is an optical component of your instrument. If your work involves trace analysis, the cell wall is a chemical reagent.
You must trust that the body is pure quartz and that the lid is inert PTFE. You must trust that the "all-quartz" label translates to real-world performance.
At KINTEK, we do not just sell glass and plastic. We provide the assurance that your "silent" variables remain silent. Whether you need the absolute transparency of quartz or the reliable utility of borosilicate, we engineer the equipment to match the rigor of your questions.
Contact Our Experts today to discuss your experimental setup. Let’s ensure your equipment isn't the variable you forgot to control.
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Electrolytic Electrochemical Cell with Five-Port
- Electrolytic Electrochemical Cell for Coating Evaluation
- Super Sealed Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
Related Articles
- The Glass Heart of the Experiment: Precision Through Systematic Care
- The Architecture of Precision: Mastering the Five-Port Water Bath Electrolytic Cell
- The Art of the Empty Vessel: Preparing Quartz Electrolytic Cells for Absolute Precision
- The Architecture of Precision: Why the Invisible Details Define Electrochemical Success
- The Silent Dialogue: Mastering Control in Electrolytic Cells




















