The Invisible Variable in Your Data
There is a distinct type of failure in experimental physics and chemistry that does not happen with a bang. It happens in silence, usually inside a dark cabinet.
We tend to obsess over the active phase of research—the voltage applied, the reagents mixed, the data captured. But the reliability of that data is often determined by what happened to your equipment weeks ago, when you weren't looking.
For a side-window optical electrolytic cell, the most dangerous time is not during the experiment. It is during storage.
An optical cell is a window into a chemical reality. If that window is compromised by a microscopic layer of salt crystallization or a chemically etched surface, your "groundbreaking anomaly" is actually just a dirty lens.
Preserving these tools requires treating storage not as a passive lack of use, but as an active defense against entropy.
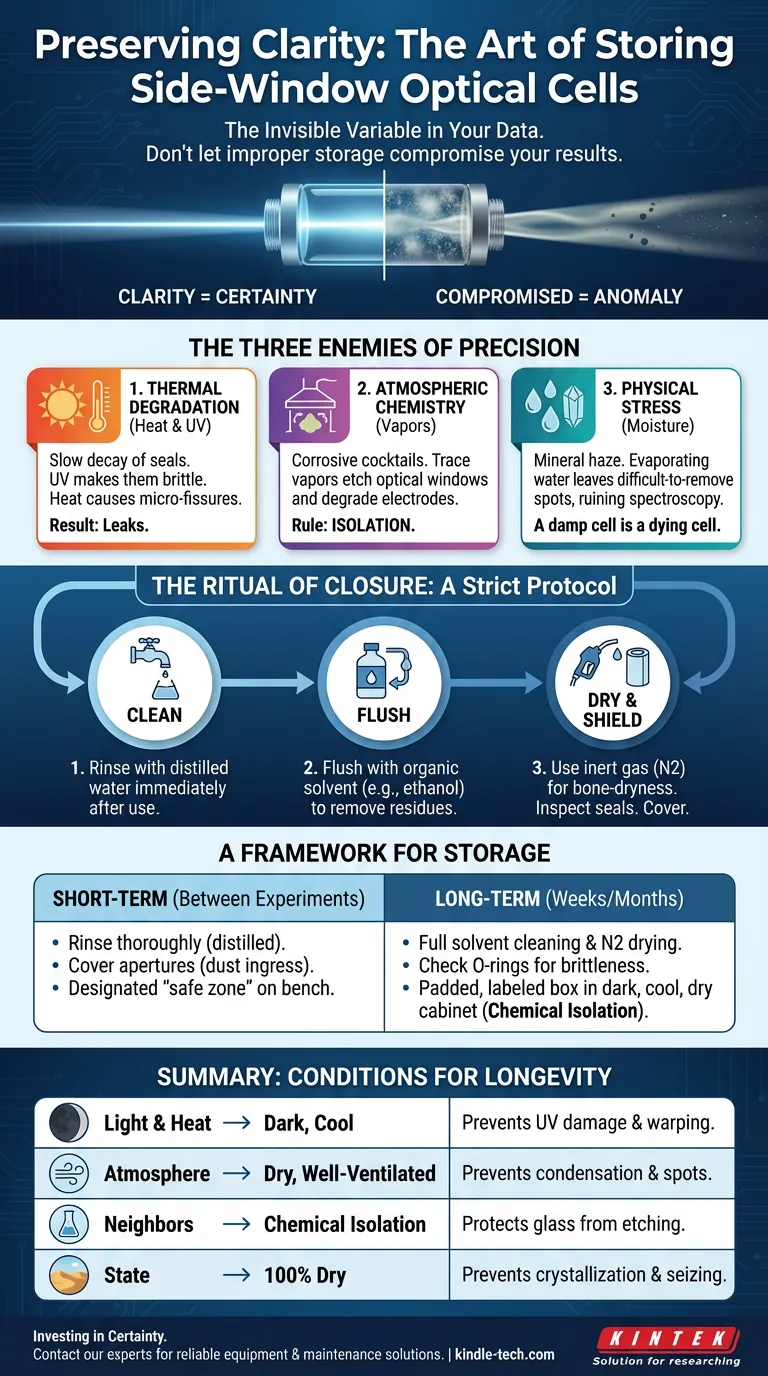
The Three Enemies of Precision
When you place a cell on a shelf, you are fighting a war against three forces: Thermal degradation, Atmospheric chemistry, and Physical stress.
If you ignore them, the cell degrades. Not immediately, but inevitably.
1. The Slow Decay of Seals (Thermal)
Your cell is likely a composite of materials—glass or quartz for the body, and PTFE or O-rings for the seals.
Glass is stubborn; it resists change. Polymers are not. High temperatures and direct UV sunlight act as slow-motion solvents for gaskets and O-rings. Over time, UV radiation makes seals brittle. Heat causes them to expand and contract, leading to micro-fissures.
When you eventually run an experiment, the electrolyte leaks. You blame the assembly. The real culprit was the windowsill you left it on three months ago.
2. The Vapor War (Chemical)
The air in a laboratory is rarely neutral. It is a cocktail of trace vapors.
If you store your optical cell in the same cabinet as harsh acids or volatile organics, you are inviting surface corruption. Corrosive vapors do not need liquid contact to do damage; over months, they can etch the optical window or degrade the electrode connections.
The rule is simple: Isolation. The cell must be physically separated from the chemicals it is designed to hold.
3. The Mineral Haze (Moisture)
A damp cell is a dying cell.
If a cell is stored with residual moisture, condensation will form. As that water evaporates, it leaves behind whatever mineral content was dissolved in it. These spots are often difficult to remove without abrasion, which ruins the optical clarity required for spectroscopy.
The Ritual of Closure
The quality of your next experiment is defined by how you finish the last one.
Storage is not just putting things away. It is a procedural reset. To ensure the longevity of a side-window optical cell, we must adhere to a strict protocol of Clean, Dry, and Shield.
The Cleaning Protocol
Never store a cell "dirty" with the intention of cleaning it later. Electrolyte salts crystallize. They seize threaded components and etch glass.
- Rinse: Use distilled water immediately after use.
- Solvent: Flush with an appropriate organic solvent (e.g., ethanol) to remove residues water cannot touch.
- Dry: This is the most critical step. Use a stream of inert gas, such as nitrogen, to ensure the cell is bone-dry.
The Inspection
Before the cell goes into the box, look at the seals. Are they pliable? Are there cracks?
Discovering a broken seal during storage is a minor annoyance. Discovering it five minutes before a critical experiment is a disaster.
A Framework for Storage
How you store the cell depends on when you need it next. The approach shifts based on the timeline.
Short-Term (Between Experiments)
- Action: Rinse thoroughly with distilled water.
- Protection: Cover apertures to prevent dust ingress.
- Location: A designated "safe zone" on the bench, away from high traffic.
Long-Term (Weeks or Months)
- Action: Full solvent cleaning and nitrogen drying.
- Inspection: Check O-rings for brittleness.
- Location: A padded, labeled box inside a dark, cool, dry cabinet. Total chemical isolation.
Summary: The Conditions for Longevity
| Variable | The Requirement | The "Why" |
|---|---|---|
| Light & Heat | Dark, cool location | Prevents UV damage to seals and thermal warping. |
| Atmosphere | Dry and Well-Ventilated | Prevents condensation and mineral spotting on optics. |
| Neighbors | Chemical Isolation | Protects glass from etching by corrosive ambient vapors. |
| State | 100% Dry | Prevents crystallization and seizing of parts. |
Investing in Certainty
There is a romance to engineering—the idea that if we treat our tools with respect, they will tell us the truth about the world.
A side-window optical electrolytic cell is a precision instrument. Its clarity is the limit of your data's accuracy. By controlling the environment in which it sleeps, you ensure it performs when it wakes.
At KINTEK, we understand that great research is built on reliable equipment. From high-purity optical cells to the consumables required to maintain them, we provide the foundation for accurate science. Don't let improper storage be the variable that ruins your results.
Contact Our Experts to discuss how to upgrade your laboratory setup or find the perfect maintenance solutions for your equipment.
Visual Guide
Related Products
- Super Sealed Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Side Window Optical Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
Related Articles
- The Glass Heart of the Experiment: Precision Through Systematic Care
- The Vessel of Truth: Why the Container Matters More Than the Chemistry
- The Architecture of Control: Mastering the Super-Sealed Electrolytic Cell
- The Anchor of Truth: Why Physical Stability Defines Electrochemical Success
- The Silence of the Seal: Why Electrochemical Precision is a Battle Against the Atmosphere



















