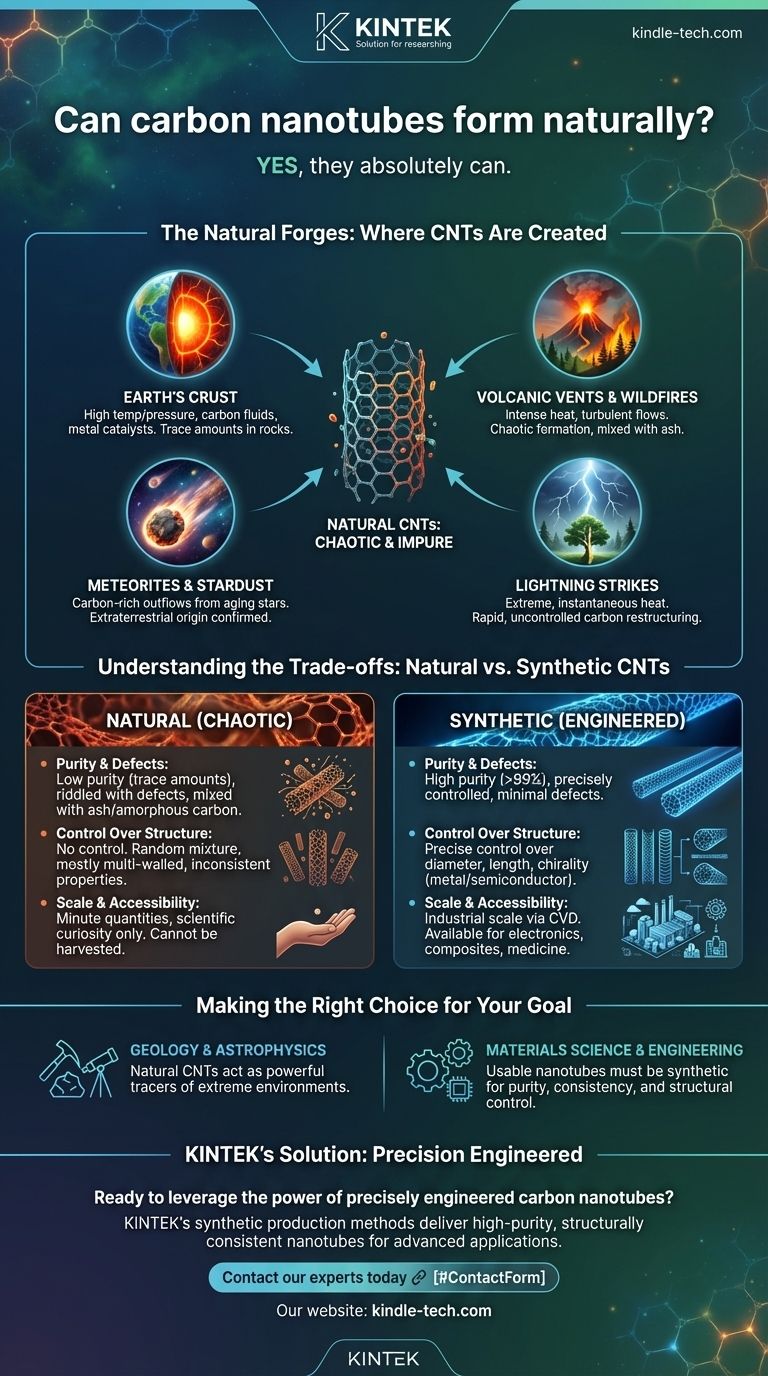
Yes, they absolutely can. Carbon nanotubes (CNTs), often perceived as a purely high-tech, lab-created material, do form naturally under specific high-energy conditions. They have been discovered in diverse environments, from deep within the Earth's crust to the remnants of distant stars, proving that nature mastered this unique carbon structure long before humanity did.
The critical distinction is not if nanotubes can form naturally, but how. Natural formation is a chaotic, uncontrolled process yielding impure, microscopic quantities, whereas industrial synthesis is a precise engineering discipline designed to produce high-purity nanotubes with specific properties for technological use.

The Natural Forges: Where CNTs Are Created
The formation of a carbon nanotube requires three key ingredients: a source of carbon, immense energy (typically high temperatures), and often a metallic catalyst. Several natural environments provide this exact recipe.
Within the Earth's Crust
Geological processes can create the necessary conditions for CNT growth. Samples from oil fields and serpentine rock formations have been found to contain naturally occurring carbon nanotubes.
These are believed to form when carbon-rich fluids like methane are subjected to high temperatures and pressures deep underground, often in the presence of natural metallic catalysts like nickel and iron particles found in the rock.
In Volcanic Vents and Wildfires
The intense heat from volcanic vents and large-scale wildfires provides the energy needed to break down carbon-containing gases and organic matter.
As these carbon atoms reassemble in the cooling, turbulent gas flows, some can organize into the hexagonal lattice of a nanotube. These natural CNTs are then ejected into the atmosphere along with ash and other particulates.
From the Cosmos: Meteorites and Stardust
Perhaps the most fascinating discovery is the presence of CNTs in meteorites, such as the famous Allende meteorite. This confirms that nanotubes can form in extraterrestrial environments.
Scientists believe they are forged in the carbon-rich outflows of aging stars. These cosmic nanotubes travel through interstellar space, eventually becoming incorporated into newly forming planetary systems and falling to Earth within meteorites.
Aftermath of Lightning Strikes
The immense energy of a lightning strike, which can reach temperatures hotter than the surface of the sun, is more than sufficient to vaporize carbonaceous material in soil or plant matter.
In the fraction of a second as the material cools, carbon atoms can restructure into various forms, including fullerenes and carbon nanotubes.
Understanding the Trade-offs: Natural vs. Synthetic CNTs
While fascinating, naturally formed CNTs are fundamentally different from their lab-grown counterparts. Understanding these differences is key to appreciating why we must synthesize them for any practical purpose.
Purity and Defects
Natural CNTs are a messy byproduct of a chaotic event. They are found in trace amounts, mixed with amorphous carbon, ash, and other minerals. They are also riddled with structural defects.
Synthetic CNTs, by contrast, are produced in highly controlled environments to achieve purities often exceeding 99%. This purity is essential for predictable electronic and mechanical performance.
Control Over Structure
Industrial synthesis allows scientists to control critical properties like the nanotube's diameter, length, and even its chirality, which determines if it behaves as a metal or a semiconductor.
Natural formation offers no such control. The resulting nanotubes are a random assortment of different types, primarily multi-walled structures with inconsistent properties.
Scale and Accessibility
Natural CNTs exist in such minuscule quantities that they are purely a scientific curiosity. They cannot be harvested or used for any application.
The entire purpose of synthetic methods, such as chemical vapor deposition (CVD), is to produce CNTs at an industrial scale, making their remarkable properties available for use in electronics, composites, and medicine.
Making the Right Choice for Your Goal
The existence of natural carbon nanotubes offers different insights depending on your field of interest. It is a testament to the universal principles of carbon chemistry, but it does not change the realities of modern technology.
- If your primary focus is geology or astrophysics: The presence of natural CNTs serves as a powerful tracer, providing clues about high-energy carbon chemistry in extreme geological and cosmic environments.
- If your primary focus is materials science or engineering: Natural CNTs are a proof of concept from nature, but all usable nanotubes for any application must be synthetic to ensure the required purity, consistency, and structural control.
Ultimately, understanding how nature creates these structures under chaotic conditions provides a profound context for our own efforts to engineer them with precision and purpose.
Summary Table:
| Natural Environment | Key Formation Conditions | Key Takeaway |
|---|---|---|
| Earth's Crust | High temp/pressure, carbon-rich fluids, metal catalysts | Found in trace amounts, proves geological formation is possible. |
| Volcanic Vents & Wildfires | Intense heat breaking down carbon matter | Forms chaotically, mixed with ash and other particulates. |
| Meteorites & Stardust | Carbon-rich outflows from aging stars | Confirms nanotubes can form in extraterrestrial environments. |
| Lightning Strikes | Extreme, instantaneous heat vaporizing carbon material | A rapid, uncontrolled process yielding various carbon forms. |
Ready to leverage the power of precisely engineered carbon nanotubes?
While nature demonstrates the possibility, KINTEK's synthetic production methods deliver the high-purity, structurally consistent nanotubes required for your advanced applications in electronics, composites, and materials science.
Contact our experts today to discuss how our lab equipment and consumables can help you achieve your research and development goals with reliable, high-performance materials.
Visual Guide
Related Products
- CVD Diamond Dressing Tools for Precision Applications
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Multi-zone Laboratory Tube Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- How plastic can be used as fuel? Turn Waste into Energy with Pyrolysis & Photo-Reforming
- What are the alternatives to FTIR? Choosing the Right Analytical Technique for Your Lab
- How does sample size affect analysis? Maximize the Reliability of Your Research
- Can one furnace have multiple zones? Achieve Customized Comfort and Energy Savings
- What is the effect of sputtering pressure? Master Atomic Energy for Superior Thin Films
- How are Ultra Freezers designed for easy movement in laboratories? Unlock Lab Flexibility with Swivel Castors
- What is the impact factor of powder metallurgy progress? A 2022 Analysis & Context
- How does sintering work in metals? A Guide to Solid-State Diffusion for Strong Parts














