In nearly all practical scenarios, yes. Heating a metal makes it temporarily weaker and more pliable while it is at an elevated temperature. However, the much more critical factor is what happens to the metal's strength after it cools, which is determined entirely by the process used.
The question isn't whether heat makes metal weaker, but rather how you use heat and subsequent cooling as a tool to achieve a desired final state. Heat unlocks the potential for change; the cooling process dictates the permanent outcome.

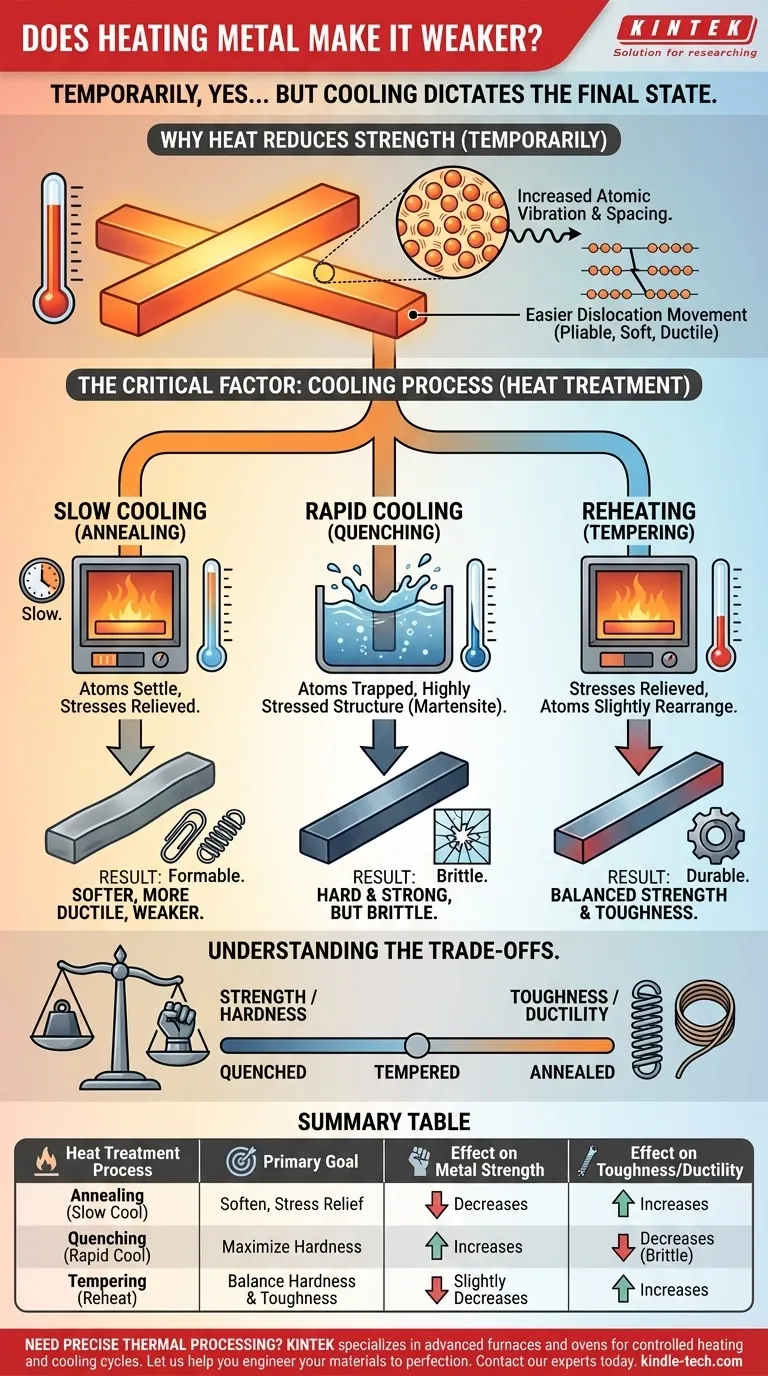
Why Heat Reduces Strength (Temporarily)
The Atomic Level Explanation
A metal's strength comes from the orderly, tightly packed crystal structure of its atoms and the strength of their metallic bonds.
When you apply heat, you are adding energy. This energy causes the atoms to vibrate more intensely, effectively creating more space between them and weakening the bonds holding them together.
The Role of Dislocations
Within this crystal structure are tiny imperfections called dislocations. The movement of these dislocations is what allows a metal to deform permanently (bend instead of break).
At higher temperatures, the increased atomic vibration makes it far easier for these dislocations to move, or "slip." This increased mobility is why a hot piece of steel is soft, ductile, and easily shaped by a blacksmith.
The Permanent Effect: How Cooling Changes Everything
The temporary weakness at high temperatures is predictable. The permanent change in properties depends entirely on how fast the metal is cooled, a process known as heat treatment.
Slow Cooling (Annealing): The Path to Softness
If you heat a metal and then allow it to cool very slowly, the atoms have ample time and energy to settle back into their most stable, lowest-energy positions.
This process, called annealing, allows the internal stresses to be relieved and the crystal grains to reform in a more perfect, orderly way. The result is a metal that is significantly softer, more ductile, and weaker than it was before. This is often done to make a metal easier to machine or form.
Rapid Cooling (Quenching): The Path to Hardness
If you heat a metal (like steel) and then cool it extremely rapidly by plunging it into water or oil, you create a dramatically different result. This is called quenching.
The atoms do not have time to return to their preferred, orderly structure. Instead, they are trapped in a highly stressed, distorted, and chaotic arrangement (for steel, this is called martensite). This new structure is extremely resistant to dislocation movement, making the metal exceptionally hard and strong, but also very brittle.
Reheating (Tempering): Finding the Balance
A quenched part is often too brittle for practical use; a sharp impact could cause it to shatter. To fix this, the part is reheated to a much lower temperature and held for a specific time.
This process, known as tempering, gives the trapped atoms just enough energy to relieve the most severe internal stresses and slightly rearrange themselves. Tempering reduces some of the extreme hardness and brittleness gained from quenching, but it adds back a crucial property: toughness. This results in a final product that is both strong and durable.
Understanding the Trade-offs
The Strength vs. Toughness Dilemma
The central trade-off in heat treatment is between strength/hardness and toughness/ductility.
- Hardness is the ability to resist scratching and indentation.
- Toughness is the ability to absorb energy and deform without fracturing.
A fully quenched piece of steel is very hard but not tough (like glass). An annealed piece is very tough but not hard (like lead). The goal of most heat treatments is to find the optimal balance between these two properties for a specific application.
The Risk of Improper Control
Heat treatment is a precise science. Overheating a metal can permanently damage its grain structure, making it weak and coarse. Cooling too slowly or too quickly can miss the desired properties entirely. The exact temperatures, times, and cooling mediums are critical to achieving the intended outcome.
Making the Right Choice for Your Goal
The effect of heat on metal is entirely dependent on your objective. The process you choose dictates the final properties.
- If your primary focus is formability or stress relief: Annealing is the correct process, intentionally making the metal softer and more workable.
- If your primary focus is maximum hardness and wear resistance: Quenching is the necessary step, but you must account for the resulting high brittleness.
- If your primary focus is a durable, high-strength component: Quenching followed by tempering provides the optimal and most common balance of properties for tools, gears, and structural parts.
Ultimately, understanding these principles transforms heat from a potential hazard into a precise instrument for engineering materials.
Summary Table:
| Heat Treatment Process | Primary Goal | Effect on Metal Strength | Effect on Metal Toughness/Ductility |
|---|---|---|---|
| Annealing (Slow Cool) | Soften, Stress Relief | Decreases | Increases |
| Quenching (Rapid Cool) | Maximize Hardness | Increases | Decreases (Increases Brittleness) |
| Tempering (Reheat Quenched Metal) | Balance Hardness & Toughness | Slightly Decreases | Increases |
Need precise thermal processing for your materials?
The principles of heat treatment are critical for achieving the exact properties your application demands. KINTEK specializes in advanced lab equipment, including furnaces and ovens, designed for controlled heating and cooling cycles. Whether your goal is maximum hardness, improved ductility, or a specific balance of strength and toughness, our solutions provide the accuracy and repeatability you need.
Let us help you engineer your materials to perfection.
Contact our thermal processing experts today to discuss your specific laboratory requirements.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is a muffle oven used for? Achieve High-Purity Heat Treatment and Analysis
- What is a muffle furnace for laboratory use? A Guide to Contaminant-Free High-Temperature Processing
- What is the difference between electric oven and muffle furnace? Choose the Right High-Temp Lab Equipment
- How do you choose calcination temperature? A Guide to Optimizing Material Properties
- What are the safety precautions for a muffle furnace? A Guide to Preventing Burns, Fires, and Electrical Hazards



















