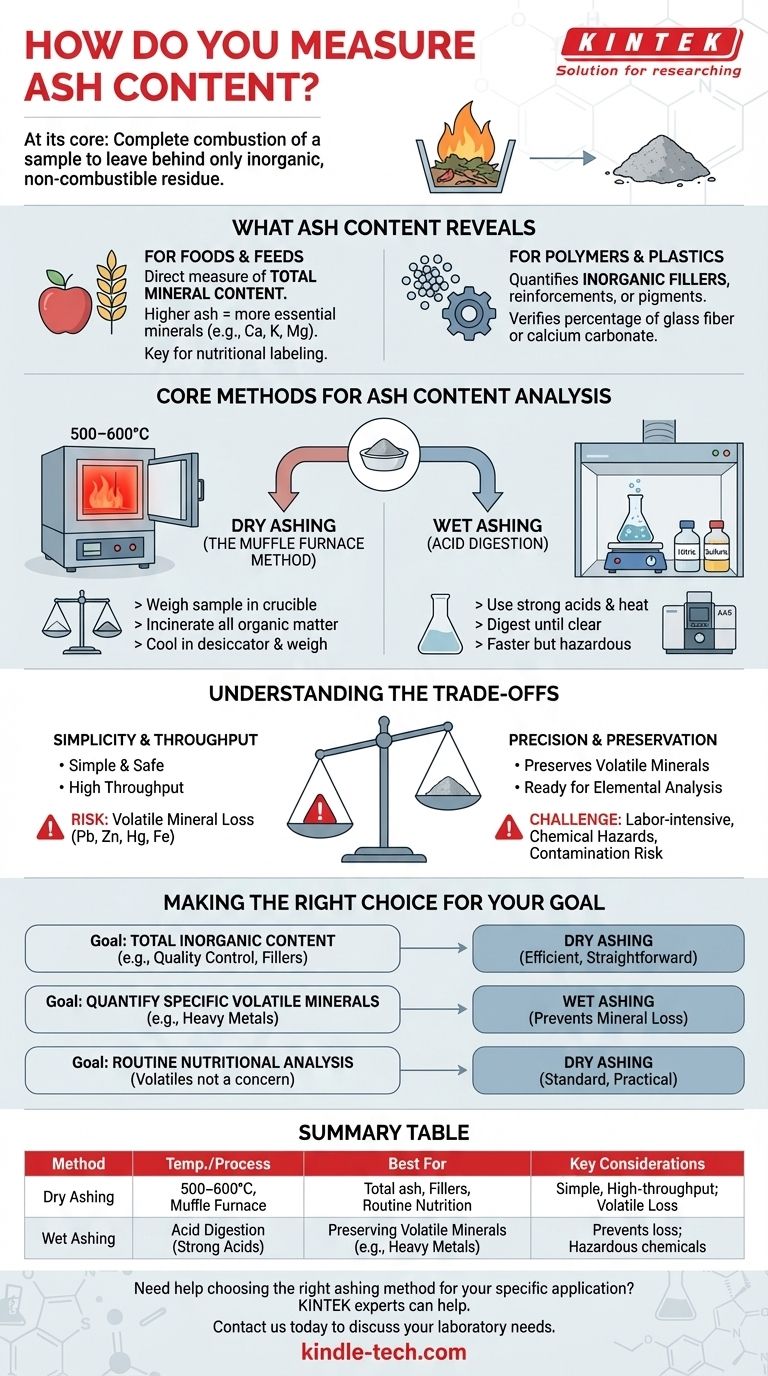
At its core, measuring ash content involves the complete combustion of a sample to burn away all organic material, leaving behind only the inorganic, non-combustible residue. This residue is then weighed to determine the ash percentage. The most common techniques for this are dry ashing, which uses a high-temperature furnace, and wet ashing, which uses acids to digest the sample.
The central challenge is not in performing the test, but in choosing the correct method. The choice between dry and wet ashing depends entirely on your sample's composition and whether you need to preserve specific volatile minerals for further analysis.

What Ash Content Reveals About Your Sample
Ash is the inorganic footprint of a material. Measuring it provides a critical data point about a sample's composition, quality, and origin.
For Foods and Feeds
In food science, ash content is a direct measure of the total mineral content. A higher ash value generally indicates a greater concentration of essential minerals like calcium, potassium, and magnesium. It is a fundamental parameter in nutritional labeling and quality control.
For Polymers and Plastics
In materials science, ash testing is used to quantify the amount of inorganic fillers, reinforcements, or pigments in a polymer. For example, it can verify the percentage of glass fiber in reinforced nylon or the amount of calcium carbonate in PVC pipes, ensuring the product meets performance specifications.
Core Methods for Ash Content Analysis
The method you choose is dictated by the sample type, the information you need, and the equipment available.
Dry Ashing (The Muffle Furnace Method)
This is the most common method. A sample is weighed into a ceramic or porcelain crucible and placed in a muffle furnace.
The furnace is heated to a high temperature, typically 500–600°C, for several hours. This incinerates all organic matter, leaving only the inorganic ash. The crucible is then cooled in a desiccator to prevent moisture absorption and weighed again. The difference in weight reveals the ash content.
Wet Ashing (Acid Digestion)
Wet ashing, also called acid digestion, is used when specific minerals need to be analyzed after the ashing process. Instead of high heat alone, this method uses strong acids (like nitric acid and sulfuric acid) and controlled heating to oxidize the organic matter.
The sample is digested in a flask until the liquid is clear. This process is faster than dry ashing but requires careful handling of hazardous chemicals and the use of a fume hood. The resulting solution is then ready for elemental analysis using techniques like Atomic Absorption Spectroscopy (AAS).
Understanding the Trade-offs
Choosing the wrong method can lead to inaccurate results, defeating the purpose of the analysis. The decision almost always comes down to a trade-off between simplicity and the preservation of volatile elements.
The Simplicity of Dry Ashing
Dry ashing is simple, safe (relative to handling strong acids), and allows for the processing of many samples simultaneously. It is the ideal choice for determining total ash content when you are not concerned with the specific mineral profile.
The Risk of Mineral Loss
The primary drawback of dry ashing is the high temperature. Volatile minerals such as lead, zinc, mercury, and iron can be partially or completely lost during incineration. If your goal is to measure these specific elements, dry ashing will produce inaccurately low results.
The Precision of Wet Ashing
Wet ashing is essential when you need to preserve volatile minerals for subsequent analysis. The lower temperatures and liquid environment prevent these elements from escaping.
This method prepares the sample directly into a liquid matrix, which is required for most advanced elemental analysis instruments. However, it is more labor-intensive, has a lower throughput, and carries the risk of chemical hazards.
The Contamination Factor
A key challenge in wet ashing is the potential for contamination from the acids themselves. Using high-purity, trace-metal-grade reagents is critical to ensure that the only elements you measure are from the sample, not your chemicals.
Making the Right Choice for Your Goal
The optimal method is dictated entirely by your final analytical objective.
- If your primary focus is determining total inorganic content for quality control (e.g., fillers in plastic): Dry ashing is the most efficient and straightforward method.
- If your primary focus is quantifying specific volatile minerals (e.g., heavy metals in a food sample): Wet ashing is required to prevent mineral loss and prepare the sample for elemental analysis.
- If your primary focus is routine nutritional analysis where volatile minerals are not a concern: Dry ashing is the standard and most practical choice.
Choosing the correct ashing technique is the first critical step toward obtaining accurate and meaningful data about your sample's composition.
Summary Table:
| Method | Temperature/Process | Best For | Key Considerations |
|---|---|---|---|
| Dry Ashing | 500–600°C in a muffle furnace | Total ash content, inorganic fillers in polymers, routine nutritional analysis | Simple, high-throughput; risk of volatile mineral loss |
| Wet Ashing | Acid digestion with strong acids | Preserving volatile minerals (e.g., heavy metals) for elemental analysis | Prevents mineral loss; requires hazardous chemical handling |
Need help selecting the right ashing method for your specific application?
At KINTEK, we specialize in providing the precise lab equipment and consumables you need for accurate ash content analysis. Whether you require a reliable muffle furnace for dry ashing or high-purity acids for wet digestion, our experts can help you choose the right tools to ensure your results are accurate and meaningful.
Contact us today to discuss your laboratory needs and discover how our solutions can enhance your analytical workflow. Get in touch via our contact form!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the material of filter press? Why Reinforced Polypropylene is the Industry Standard
- What are the safety precautions when performing heat treatment? A Guide to Avoiding Burns and Hazards
- Is sintering the same as welding? Key Differences in Material Bonding and Fusion Explained
- What are types of pharmaceutical mixers? Choose the Right Mixer for Your Formulation
- What is the difference between oven and furnace in laboratory? Choose the Right Thermal Tool for Your Lab
- What are the elements of bio-oil? Unlocking the Chemistry of Renewable Fuel
- How does sample size affect analysis? Maximize the Reliability of Your Research
- What affects sputtering yield? Master the Physics for Maximum Deposition Efficiency



















