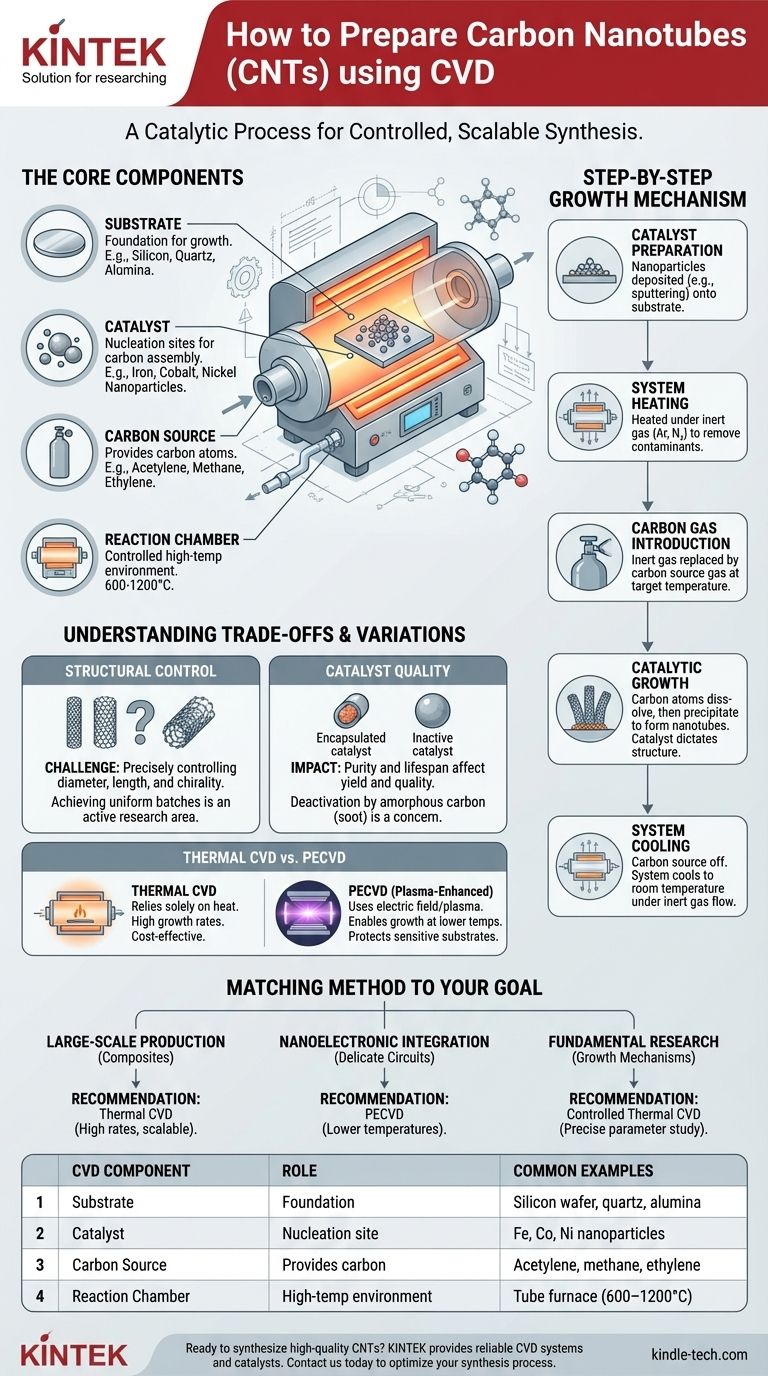
In short, preparing carbon nanotubes (CNTs) via Chemical Vapor Deposition (CVD) involves heating a substrate coated with metal catalyst nanoparticles inside a reaction chamber and introducing a carbon-containing gas. The hot catalyst particles break down the gas, and the carbon atoms reassemble on the catalyst's surface, precipitating out to form the cylindrical, hollow structure of a carbon nanotube. This catalytic process is the key to growing high-quality CNTs at relatively low temperatures.
The core principle is not just heating a gas, but using a nanoscale metal catalyst as a "seed" for growth. The catalyst dictates where the nanotubes form and critically influences their structure, making CVD a highly controllable and scalable synthesis method.

The Core Components of the CVD Process
To understand how CVD works for CNT synthesis, it's essential to understand the four key components involved in the reaction.
The Substrate
The substrate is the physical foundation upon which the carbon nanotubes will grow. It is typically a flat material, such as a silicon wafer, quartz, or alumina, that can withstand high temperatures. The choice of substrate often depends on the final application of the CNTs.
The Catalyst
The catalyst is the most critical element in the process. It consists of nanoparticles of a transition metal, most commonly iron, cobalt, or nickel. These particles act as nucleation sites, triggering the decomposition of the carbon gas and guiding the assembly of carbon atoms into the nanotube structure.
Without a catalyst, the temperatures required to break down the carbon source gas would be prohibitively high. The catalyst dramatically lowers this required temperature.
The Carbon Source
The carbon source, or precursor, is a hydrocarbon gas that provides the carbon atoms needed to build the nanotubes. Common choices include acetylene (C₂H₂), ethylene (C₂H₄), methane (CH₄), or even alcohols like ethanol. The flow rate and type of gas are key variables for controlling the growth rate and quality of the CNTs.
The Reaction Chamber
This is a furnace or tube that provides a controlled, high-temperature environment. The chamber is first purged with an inert gas (like argon or nitrogen) to remove oxygen and then heated to the target synthesis temperature, typically between 600°C and 1200°C.
The Step-by-Step Growth Mechanism
The synthesis of CNTs via CVD follows a clear sequence of events.
Step 1: Catalyst Preparation
First, the catalyst nanoparticles are deposited onto the substrate. This can be done through various methods, such as sputtering or evaporating a thin metal film which, upon heating, breaks up into nanoscale droplets.
Step 2: System Heating
The substrate is placed inside the reaction chamber, which is then sealed and heated to the desired growth temperature under a continuous flow of an inert gas. This step ensures the environment is stable and free of reactive contaminants before synthesis begins.
Step 3: Carbon Gas Introduction
Once the target temperature is reached, the inert gas flow is partially or fully replaced by the carbon source gas. This marks the beginning of the growth phase.
Step 4: Catalytic Growth
As the carbon source gas flows over the hot catalyst nanoparticles, the gas molecules decompose. Carbon atoms dissolve into the metal nanoparticle until it becomes supersaturated. To relieve this saturation, the carbon precipitates out from the surface of the particle, forming the stable, cylindrical lattice of a carbon nanotube.
Step 5: System Cooling
After a set period of growth, the carbon source gas is turned off, and the system is cooled down to room temperature under the flow of an inert gas. The substrate, now coated with a "forest" of carbon nanotubes, can be safely removed.
Understanding the Trade-offs and Variations
While CVD is a powerful technique, it is essential to understand its nuances and common variations.
The Challenge of Structural Control
A significant challenge in CNT synthesis is precisely controlling the final structure—such as diameter, length, and electronic properties (chirality). While the process is highly reproducible for producing CNTs in general, achieving uniform batches with identical properties remains an area of active research.
Thermal CVD vs. Plasma-Enhanced CVD (PECVD)
Thermal CVD, described above, relies solely on heat to drive the reaction. A common variation is Plasma-Enhanced CVD (PECVD), which uses an electric field to generate a plasma. This plasma helps break down the carbon source gas more efficiently, allowing CNT growth at even lower temperatures. This is particularly valuable when depositing CNTs onto temperature-sensitive substrates, such as those used in integrated electronics.
Catalyst Quality and Yield
The purity and lifespan of the catalyst directly impact the quality and yield of the CNTs. Over time, the catalyst particles can become encapsulated in amorphous carbon (non-structured soot) or other byproducts, which deactivates them and stops nanotube growth. Optimizing gas flow and temperature is crucial to maximize catalyst lifetime.
Matching the Method to Your Goal
The specific parameters of the CVD process should be tailored to your intended outcome.
- If your primary focus is large-scale production for composite materials: Standard thermal CVD is often the most cost-effective method due to its high growth rates and scalability.
- If your primary focus is integration with nanoelectronic devices: PECVD is the superior choice because its lower processing temperatures prevent damage to delicate, pre-existing electronic circuits on the substrate.
- If your primary focus is fundamental research on growth mechanisms: A highly controlled thermal CVD system is ideal, as it allows for the precise and systematic study of how individual parameters like temperature and catalyst type affect nanotube formation.
By mastering these core principles, you can effectively leverage CVD to synthesize carbon nanotubes for a vast range of advanced applications.
Summary Table:
| CVD Component | Role in CNT Synthesis | Common Examples |
|---|---|---|
| Substrate | Foundation for growth | Silicon wafer, quartz, alumina |
| Catalyst | Nucleation site for carbon assembly | Iron, cobalt, nickel nanoparticles |
| Carbon Source | Provides carbon atoms | Acetylene, methane, ethylene |
| Reaction Chamber | Controlled high-temperature environment | Tube furnace (600–1200°C) |
Ready to synthesize high-quality carbon nanotubes for your research or application? KINTEK specializes in lab equipment and consumables, providing reliable CVD systems and catalysts tailored to your laboratory needs. Whether you're scaling up production or integrating CNTs into delicate electronics, our expertise ensures precise control over growth parameters. Contact us today to discuss how our solutions can optimize your CNT synthesis process!
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
People Also Ask
- Why is a high-purity alumina lining required for high-temperature tube furnaces? Ensure Accurate Biomass Research
- What is the role of corundum tubes in oxygen permeation testing? Ensure Integrity for Bi-doped Membranes
- Why is an Alumina Ceramic Tube Support Necessary for 1100°C Experiments? Ensure Data Accuracy and Chemical Inertness
- What tube is used for tubular furnace? Choose the Right Material for Temperature & Atmosphere
- What is the pressure on a tube furnace? Essential Safety Limits for Your Lab



















