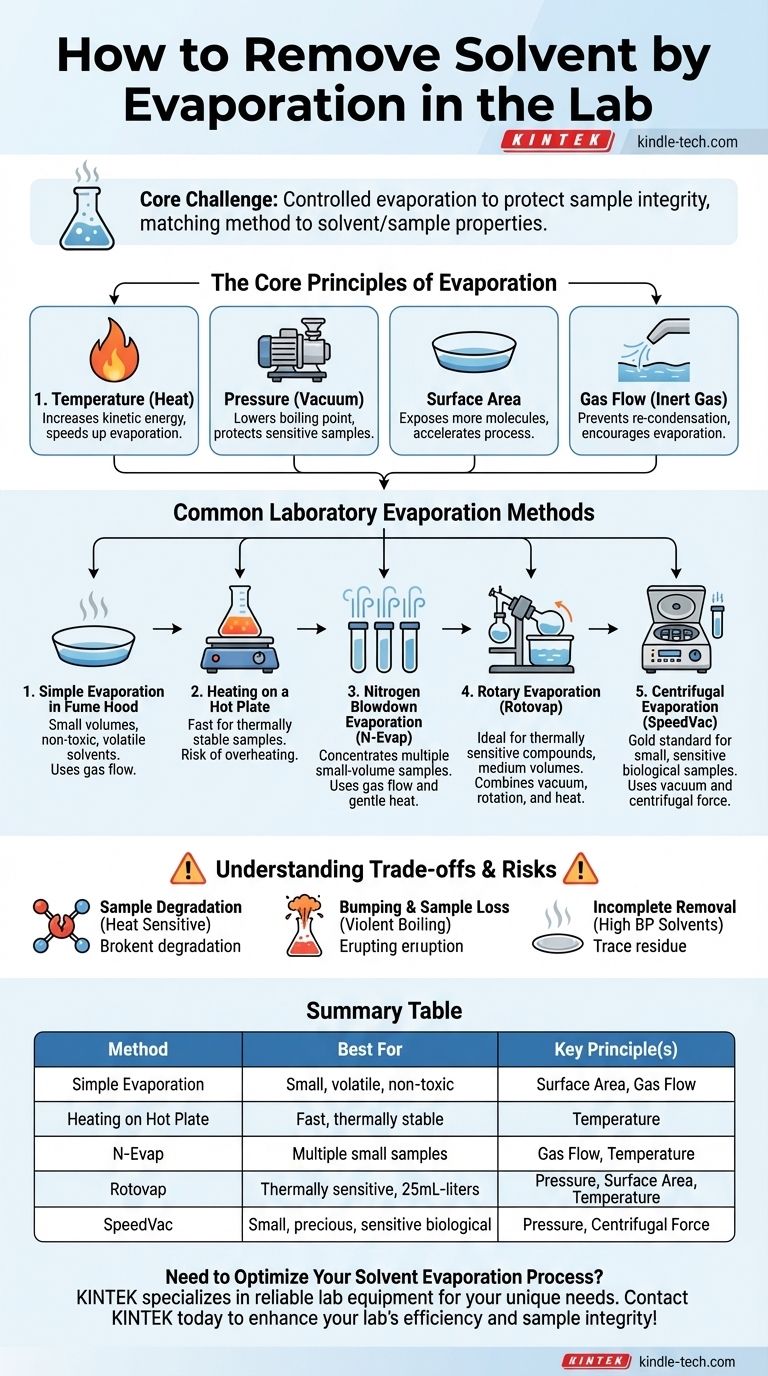
In a laboratory setting, removing a solvent by evaporation involves applying energy, typically as heat, and/or reducing the ambient pressure. This gives the solvent molecules enough energy to escape into the gas phase, leaving behind the less volatile components of your mixture, such as your desired product. This process is actively managed using specific equipment to control the speed and temperature.
The core challenge of solvent removal is not just evaporation, but controlled evaporation. Your goal is to remove the solvent as quickly as possible without degrading or losing the sample you are trying to isolate. The best method always depends on the solvent's properties and the sample's sensitivity.

The Core Principles of Evaporation
To choose the right method, you must first understand the four factors you can manipulate to control the speed of evaporation. These principles are the foundation of every technique.
The Role of Temperature
Increasing the temperature of a solvent directly increases the kinetic energy of its molecules. This makes it easier for them to overcome the forces holding them in the liquid phase and escape as a gas. More heat means faster evaporation.
The Role of Pressure
Reducing the pressure above a liquid lowers its boiling point. This is the single most important principle for handling sensitive samples. By placing a sample under vacuum, you can make the solvent boil and evaporate at a much lower, safer temperature, protecting your compound from thermal degradation.
The Role of Surface Area
Evaporation only occurs at the surface of the liquid. By increasing the surface area, you expose more solvent molecules to the gas phase, dramatically speeding up the process. This is why a puddle evaporates faster than the same amount of water in a deep cup.
The Role of Gas Flow
When a solvent evaporates, it creates a layer of vapor just above the liquid's surface, which can lead to re-condensation. By blowing a steady stream of an inert gas (like nitrogen) over the surface, you constantly push this vapor away, encouraging more of the liquid to evaporate to restore equilibrium.
Common Laboratory Evaporation Methods
These principles are applied using several standard pieces of laboratory equipment, each suited for different scales and sample types.
Simple Evaporation in a Fume Hood
This is the most basic method. The sample is placed in a wide, shallow container (like an evaporating dish or watch glass) inside a fume hood. The hood's airflow provides a gentle gas flow to carry away vapors.
This method is only suitable for very small volumes of non-toxic, highly volatile solvents and for samples that are not sensitive to air or prolonged exposure.
Heating on a Hot Plate
A simple way to accelerate evaporation is by gently heating the sample on a hot plate in a fume hood. This directly applies the principle of increasing temperature.
While fast, this method offers poor temperature control and creates a high risk of overheating or "bumping" (violent boiling), which can lead to sample loss and degradation.
Nitrogen Blowdown Evaporation
Also known as N-Evap, this technique uses a manifold to direct multiple fine streams of nitrogen gas onto the surface of samples in vials or a microplate. It often includes gentle heating from a block or water bath below.
This is highly effective for concentrating multiple small-volume samples simultaneously and is common in sample preparation for analytical chemistry.
Rotary Evaporation ("Rotovap")
The rotary evaporator is the workhorse of the synthetic chemistry lab. It combines all principles for efficient and gentle solvent removal.
- Reduced Pressure: A vacuum pump lowers the boiling point.
- Increased Surface Area: The flask is continuously rotated, coating the inner walls with a thin film of the sample.
- Gentle Heating: The rotating flask sits in a water bath for stable, controlled heating.
This method is ideal for thermally sensitive compounds and for removing solvent from volumes typically ranging from 25 mL to several liters.
Centrifugal Evaporation ("SpeedVac")
A centrifugal evaporator (often called a SpeedVac) places samples under a deep vacuum while spinning them in a centrifuge. The centrifugal force prevents bumping, and gentle heat is applied via infrared radiation.
This is the gold standard for safely concentrating many small, precious, or sensitive samples, such as DNA, RNA, or peptides, without any risk of cross-contamination or loss.
Understanding the Trade-offs and Risks
Choosing a method requires balancing speed against potential problems. Being aware of these trade-offs is critical for success.
Sample Degradation
The primary risk is thermal degradation. Many organic compounds and nearly all biological molecules can be destroyed by excessive heat. This is why methods that use reduced pressure, like rotary evaporation, are so essential.
Bumping and Sample Loss
When a liquid is heated under vacuum without agitation, it can superheat and then boil violently in a single, large eruption. This phenomenon, called bumping, can cause you to lose a significant portion of your sample into the vacuum system. Rotation (in a rotovap) or centrifugal force (in a SpeedVac) are used specifically to prevent this.
Incomplete Solvent Removal
High-boiling point solvents like DMSO or DMF can be difficult to remove completely. Even under a strong vacuum, trace amounts may remain, contaminating your final product. This can sometimes be addressed by adding a more volatile solvent (like toluene) and re-evaporating, a process known as azeotropic drying.
Making the Right Choice for Your Sample
Your decision should be guided by your sample's characteristics and your final goal.
- If your sample is thermally stable and the solvent is volatile: Simple heating in a fume hood or nitrogen blowdown may be sufficient and fast.
- If your sample is thermally sensitive or in a volume greater than 20 mL: A rotary evaporator is the standard and most reliable choice.
- If you have many small, highly sensitive biological samples: A centrifugal evaporator offers the highest level of protection and efficiency.
- If your goal is to isolate a product with maximum purity: Always choose a method with vacuum to use the lowest possible temperature, minimizing the formation of thermal byproducts.
By matching the technique to the needs of your sample, you ensure efficient solvent removal while maximizing the integrity and yield of your final product.
Summary Table:
| Method | Best For | Key Principle(s) |
|---|---|---|
| Simple Evaporation | Small volumes of non-toxic, volatile solvents | Surface Area, Gas Flow |
| Heating on a Hot Plate | Fast evaporation of thermally stable samples | Temperature |
| Nitrogen Blowdown (N-Evap) | Concentrating multiple small-volume samples | Gas Flow, Temperature |
| Rotary Evaporation (Rotovap) | Thermally sensitive compounds; volumes from 25mL to liters | Pressure, Surface Area, Temperature |
| Centrifugal Evaporation (SpeedVac) | Small, precious, or sensitive biological samples | Pressure, Centrifugal Force |
Need to Optimize Your Solvent Evaporation Process?
Choosing the right equipment is critical for protecting your sensitive samples and maximizing yield. KINTEK specializes in providing reliable lab equipment and consumables tailored to your laboratory's unique needs.
Whether you require a gentle rotary evaporator for your synthetic chemistry workflows or a high-throughput centrifugal evaporator for precious biological samples, we have the solution.
Let our experts help you enhance your lab's efficiency and sample integrity.
Contact KINTEK today to find the perfect evaporation system for your application!
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- What are some common uses of freeze drying? Preserve Delicate Materials with Precision
- What role does freeze drying play in scientific research? Preserve Sample Integrity for Reliable Results
- What is the freeze drying or lyophilisation process? A Guide to Gentle, High-Quality Dehydration
- Why are laboratory freeze dryers considered economical tools? Maximize Value and Minimize Loss
- What are the main steps involved in the freeze-drying process? A Guide to the 3 Key Stages



















