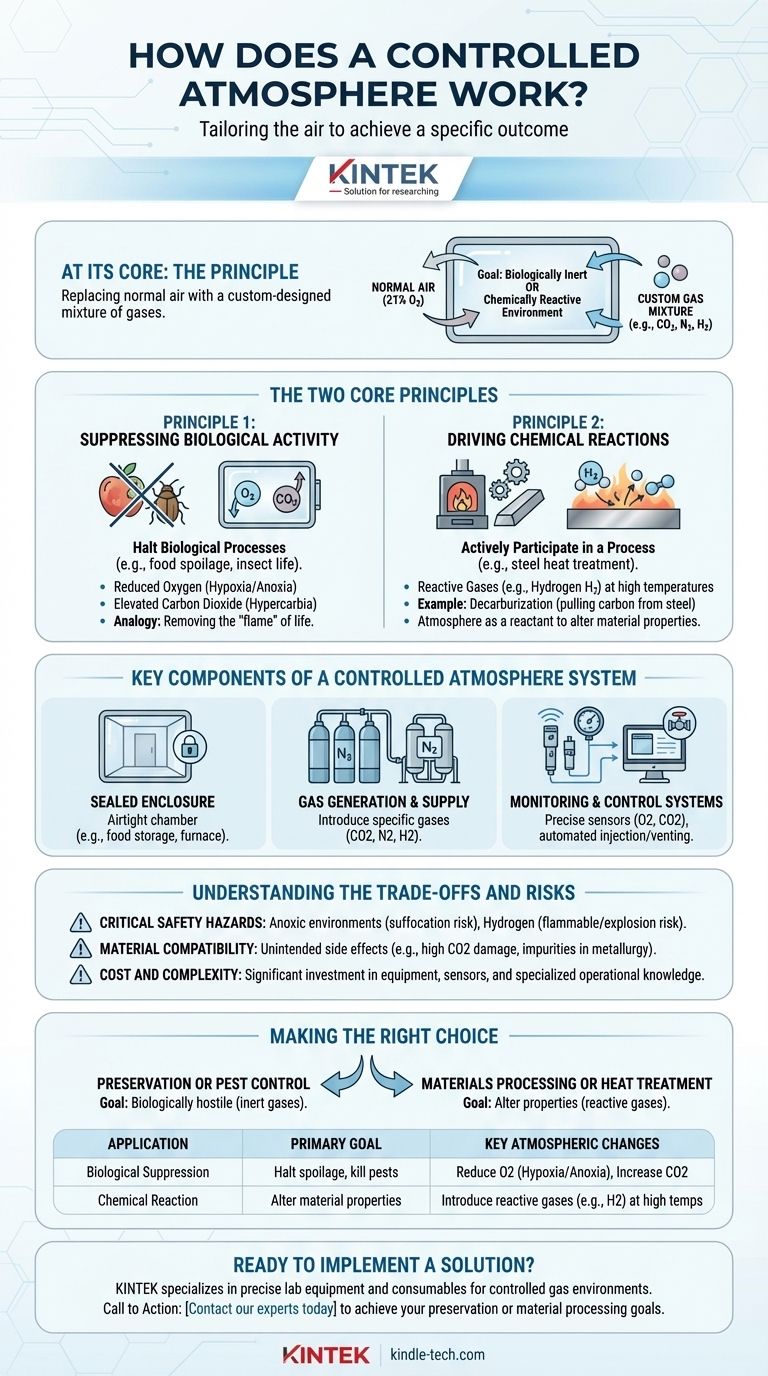
At its core, a controlled atmosphere works by precisely replacing the normal air in a sealed environment with a specific, custom-designed mixture of gases. This is accomplished by actively managing the levels of gases like oxygen (O2), carbon dioxide (CO2), and hydrogen (H2) to create an environment that is either biologically inert or chemically reactive, depending on the goal.
Controlling an atmosphere isn't about one specific gas mixture; it's the principle of tailoring the air to achieve a specific outcome. This custom blend is designed to either halt biological processes, like food spoilage and insect life, or to drive specific chemical reactions, such as those in metal treatment.

The Two Core Principles of Atmospheric Control
The function of a controlled atmosphere depends entirely on its intended purpose. The two primary applications—biological suppression and chemical reaction—operate on fundamentally different principles.
Principle 1: Suppressing Biological Activity
Normal air, with its ~21% oxygen content, supports life and drives decay. By altering this, we can effectively press "pause" on these biological processes.
This is achieved by creating an atmosphere with reduced oxygen (hypoxia or anoxia) and often elevated carbon dioxide (hypercarbia). This mixture is lethal to insects and dramatically slows the respiration of fruits and vegetables, extending their freshness.
Think of it like putting out a fire. By removing the oxygen, you remove the key element required for the "flame" of life and decay to continue burning.
Principle 2: Driving Chemical Reactions
In industrial settings like furnaces, gases are not used to suppress a process but to actively participate in one. At high temperatures, certain gases become powerful chemical agents.
For example, in steel heat treatment, an atmosphere rich in hydrogen (H2) can be used. The hydrogen actively pulls carbon atoms out of the steel (a process called decarburization) or strips oxygen atoms from iron oxide, reducing it back to pure iron.
In this context, the controlled atmosphere acts as a reactant. The specific gas composition is chosen to force a predictable chemical change and achieve desired material properties, such as hardness or purity.
Key Components of a Controlled Atmosphere System
Creating and maintaining these precise gas mixtures requires a system of specialized components working in concert.
The Sealed Enclosure
The first requirement is an airtight chamber. This could be a food storage room, a shipping container, a museum display case, or a high-temperature industrial furnace. If the enclosure leaks, the controlled atmosphere cannot be maintained.
Gas Generation and Supply
The specific gases must be introduced into the enclosure. This is often done with industrial gas tanks (CO2, N2), on-site nitrogen generators that separate nitrogen from the air, or controlled injections of reactive gases like hydrogen.
Monitoring and Control Systems
This is the "controlled" part of the process. Sophisticated sensors constantly measure the exact concentration of key gases like O2 and CO2. These sensors feed data to an automated system that injects or vents gases as needed to maintain the desired levels with high precision.
Understanding the Trade-offs and Risks
While powerful, controlled atmosphere technology is not without its challenges and inherent dangers.
Critical Safety Hazards
An atmosphere designed to be lethal to insects is also lethal to humans. Environments with low oxygen (anoxic) present a severe suffocation hazard, as a person can lose consciousness in seconds without warning. Atmospheres using hydrogen are highly flammable and pose an explosion risk.
Material Compatibility
The chosen gas mixture can have unintended side effects. For instance, high CO2 levels can cause physiological damage to certain types of produce. In metallurgy, an incorrect gas balance can introduce impurities or create brittle spots, ruining the final product.
Cost and Complexity
These are not simple systems. They require a significant capital investment in equipment, sensors, and control logic. Furthermore, they demand specialized knowledge to operate safely and effectively, adding to the operational cost.
Making the Right Choice for Your Goal
Your application dictates the entire approach to atmospheric control.
- If your primary focus is preservation or pest control: Your goal is to create a biologically hostile environment by displacing oxygen with inert gases like nitrogen or carbon dioxide.
- If your primary focus is materials processing or heat treatment: You are using specific gases like hydrogen as active chemical agents to alter the fundamental properties of a material at high temperatures.
Ultimately, mastering a controlled atmosphere means understanding your goal and choosing the right gases to either stop a process or start one.
Summary Table:
| Application | Primary Goal | Key Atmospheric Changes |
|---|---|---|
| Biological Suppression | Halt spoilage, kill pests | Reduce O2 (Hypoxia/Anoxia), Increase CO2 |
| Chemical Reaction | Alter material properties | Introduce reactive gases (e.g., H2) at high temperatures |
Ready to implement a controlled atmosphere solution for your laboratory or industrial process? KINTEK specializes in the precise lab equipment and consumables needed to generate, monitor, and control specialized gas environments safely and effectively. Contact our experts today to discuss how we can help you achieve your preservation or material processing goals.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Vertical Laboratory Tube Furnace
People Also Ask
- What is hydrogen annealing? The Complete Guide to Bright Annealing
- How are inert gases utilized in the thermal treatment of metals? Protect Your Alloys with Nitrogen & Argon Atmospheres
- What is the core function of a high-temperature atmosphere sintering furnace in the fabrication of Ni-Al2O3-TiO2 composites?
- How does an atmosphere furnace facilitate the post-treatment of nickel-plated carbon fibers? Ensure Peak Bonding
- What is the role of an Atmosphere Furnace in the preparation of lignin-based graphene oxide? Key Carbonization Insights
- What is the use of hydrogen furnace? Achieve Superior Purity in High-Temperature Processing
- Why is high-purity argon needed for 12Kh18N10T steel processing? Protect Your Surface Integrity and Data Reliability
- What is the main function of an inert atmosphere? Protecting Materials from Oxidation and Degradation



















