For laboratory applications, the body of an electrolysis cell is typically constructed from high borosilicate glass, often with a wall thickness around 4.5 to 5 mm. This material is chosen for its chemical inertness and thermal resistance. The physical container, however, is only one part of a larger system designed to drive a chemical reaction using electricity.
The cell body is simply an inert container. The true function of an electrolytic cell is defined by its three core internal components: two electrodes (anode and cathode), an electrolyte containing mobile ions, and an external power source to drive the reaction.

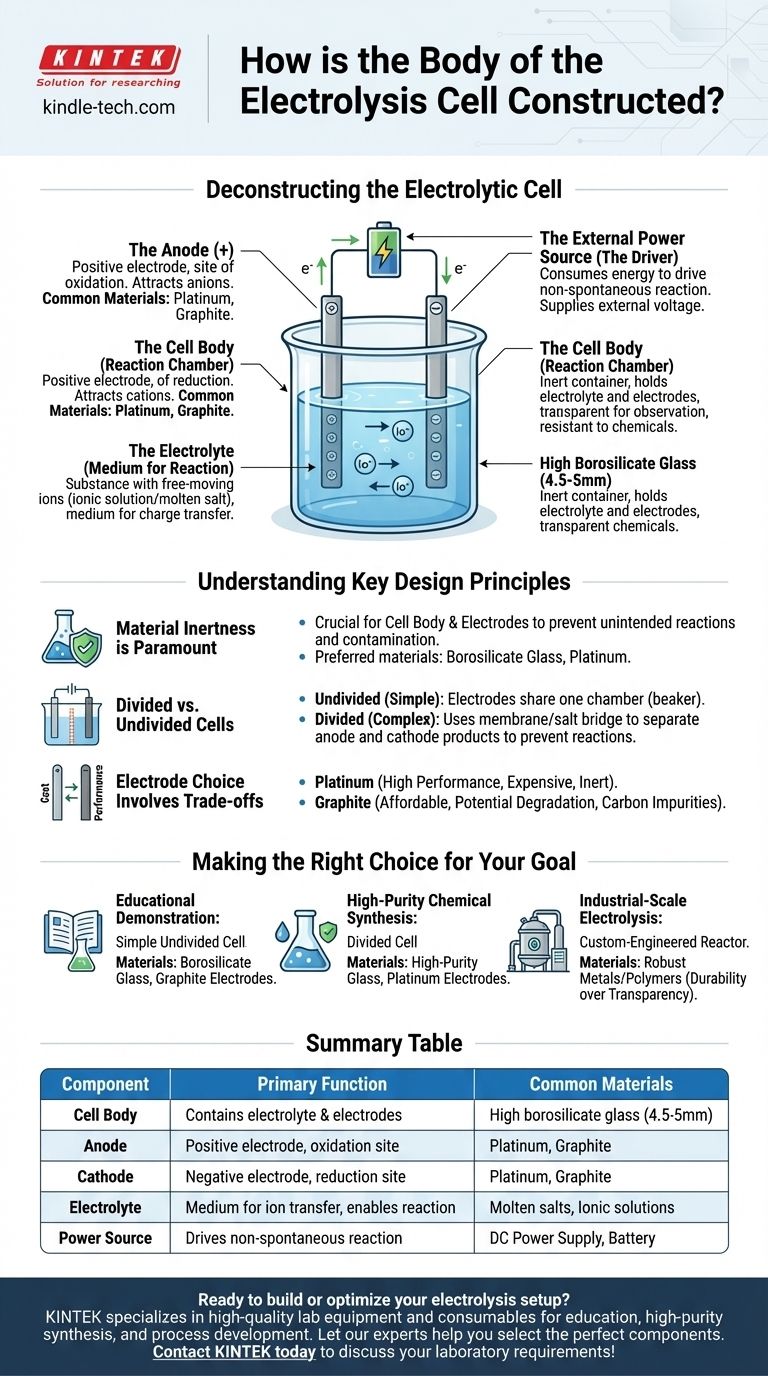
Deconstructing the Electrolytic Cell
To understand the cell's construction, you must look beyond the physical vessel and analyze the functional components it houses. An electrolytic cell is a complete system where each part plays a critical role in the process of electrolysis.
The Cell Body (The Reaction Chamber)
The body's primary function is to contain the electrolyte and hold the electrodes in place without interfering with the chemical reaction.
High borosilicate glass is a common material because it is transparent, allowing for observation, and highly resistant to corrosion from the often-aggressive chemicals used as electrolytes. For specific applications, other inert materials like certain polymers may be used.
The Electrodes (The Anode and Cathode)
Electrodes are the conductors that introduce electrical energy into the system. An electrolytic cell always has two.
- The Anode is the positive electrode. It attracts negatively charged ions (anions) and is where oxidation occurs.
- The Cathode is the negative electrode. It attracts positively charged ions (cations) and is where reduction occurs.
These are typically made from inert materials, such as platinum or graphite, which conduct electricity well but do not readily participate in the chemical reaction themselves.
The Electrolyte (The Medium for Reaction)
The electrolyte is the substance that contains free-moving ions and fills the cell body. It is the medium through which charge is transferred between the electrodes.
Electrolytes can be an ionic compound dissolved in a solvent (like salt in water) or a molten ionic compound (like molten sodium chloride). The specific ions within the electrolyte are what will be oxidized or reduced during electrolysis.
The External Power Source (The Driver)
Unlike a battery that produces energy, an electrolytic cell consumes energy to force a non-spontaneous reaction to occur.
This energy is supplied by an external power source, such as a battery or a DC power supply, which is connected to the anode and cathode. This external voltage is what pushes electrons through the circuit and drives the ions to their respective electrodes.
Understanding Key Design Principles
The construction of an electrolytic cell is guided by fundamental principles that ensure its proper function. Misunderstanding these can lead to failed experiments or inefficient processes.
Material Inertness is Paramount
The single most important principle for the cell body and electrodes is chemical inertness. The container and the electrical conductors must not react with the electrolyte. Any unintended reaction can contaminate the products and disrupt the desired electrochemical process. This is why specialized materials like borosilicate glass and platinum are favored over standard glass or reactive metals.
Divided vs. Undivided Cells
The simplest cell design is an "undivided" cell, where both electrodes share a common electrolyte in a single chamber, like a beaker.
However, sometimes the products formed at the anode and cathode can react with each other. In these cases, a "divided" cell is used. This design incorporates a porous membrane or a salt bridge to separate the cell into two distinct half-cells, keeping the products isolated while still allowing ions to flow between the compartments.
Electrode Choice Involves Trade-offs
While both are common, the choice between platinum and graphite electrodes involves a classic cost-versus-performance trade-off.
Platinum is extremely inert and efficient but very expensive. Graphite is a much more affordable conductor but can degrade or react under certain conditions, especially at high voltages or with specific electrolytes, potentially introducing carbon impurities into the system.
Making the Right Choice for Your Goal
The optimal construction of an electrolytic cell depends entirely on its intended application.
- If your primary focus is educational demonstration: A simple, undivided beaker made of borosilicate glass with inexpensive graphite electrodes is perfectly sufficient and cost-effective.
- If your primary focus is high-purity chemical synthesis: A divided cell made from high-purity glass with stable, inert platinum electrodes is essential to prevent product contamination and side reactions.
- If your primary focus is industrial-scale electrolysis: The cell will be a highly specialized, custom-engineered reactor, often built from robust metals or polymers designed for durability, efficiency, and continuous operation rather than transparency.
Understanding these core components and their functions empowers you to select or design a cell that precisely matches your objective.
Summary Table:
| Component | Primary Function | Common Materials |
|---|---|---|
| Cell Body | Contains the electrolyte and electrodes | High borosilicate glass (4.5-5mm) |
| Anode | Positive electrode; site of oxidation | Platinum, Graphite |
| Cathode | Negative electrode; site of reduction | Platinum, Graphite |
| Electrolyte | Medium for ion transfer; enables reaction | Molten salts, Ionic solutions |
| Power Source | Drives the non-spontaneous reaction | DC Power Supply, Battery |
Ready to build or optimize your electrolysis setup? The right cell construction is critical for your lab's success, whether for education, high-purity synthesis, or process development. KINTEK specializes in providing the high-quality lab equipment and consumables you need—from durable borosilicate glassware to inert platinum and graphite electrodes. Let our experts help you select the perfect components for your specific application. Contact KINTEK today to discuss your laboratory requirements!
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Double-Layer Water Bath Electrolytic Electrochemical Cell
People Also Ask
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control
- What are the key features of the five-port water bath electrolytic cell? Precision Control for Electrochemical Experiments
- What are the standard aperture specifications of the electrolytic cell? Key Sizes for Your Electrochemical Setup
- What are the standard aperture sizes on the lid of the multifunctional electrolytic cell? Key Ports for Your Electrochemical Setup
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup



















