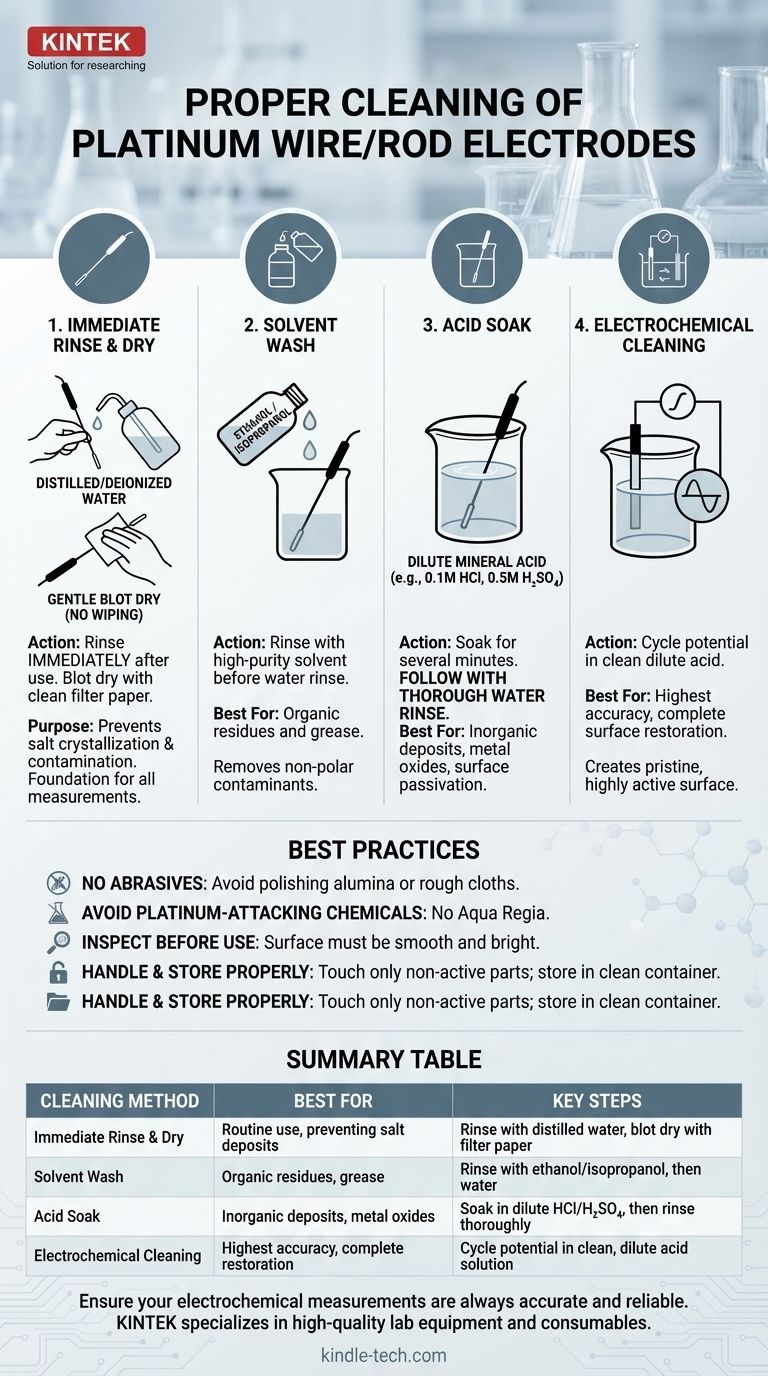
To properly clean a platinum wire or rod electrode after use, you must act promptly. The standard procedure is to immediately remove the electrode from the electrolyte, rinse it thoroughly with distilled or deionized water, and then gently blot it dry with clean filter paper. This simple process prevents residual electrolyte from drying on the surface, which can lead to contamination and the formation of passive, non-reactive layers.
Your goal in cleaning an electrode is not just tidiness; it is to restore a chemically pure and electrochemically active platinum surface. Proper cleaning is the most critical step in ensuring the accuracy and reproducibility of your subsequent measurements.

The Foundation: Immediate Post-Use Cleaning
This basic protocol should be performed after every single use. It is the first line of defense against contamination and performance degradation.
Step 1: Prompt Removal and Rinsing
As soon as the experiment is complete, remove the electrode from the solution. Rinse the platinum surface generously with a stream of distilled or deionized water from a wash bottle. Ensure all surfaces that were in contact with the electrolyte are cleaned.
Step 2: Gentle Drying
Use a fresh piece of laboratory-grade filter paper to gently blot the moisture from the electrode surface. Do not wipe or rub the platinum, as it is a soft metal and can be easily scratched or contaminated by fibers. The goal is simply to absorb the water.
Why This Is Critical
Performing this simple rinse-and-dry procedure immediately prevents salts from the electrolyte from crystallizing on the surface. It also washes away loosely adsorbed species before they have a chance to bond more permanently or react to form stubborn films.
Addressing Contamination: From Simple to Stubborn
If the basic cleaning procedure is insufficient, or if you suspect specific types of contamination, more aggressive methods are required.
For Organic Residues and Grease
If the electrode was used in a solution containing organic compounds, or if you suspect it has been contaminated with grease from handling, a solvent wash is necessary. Rinsing the electrode with a high-purity solvent like ethanol or isopropanol before the water rinse is highly effective.
For Inorganic Deposits or Metal Oxides
To remove stubborn inorganic salts, metal deposits, or surface oxides that can passivate the platinum, a chemical treatment is required. Soaking the electrode in dilute mineral acid (e.g., 0.1M HCl or 0.5M H₂SO₄) for several minutes is a standard procedure. Always follow an acid wash with a thorough rinse with deionized water.
For a Complete Surface Restoration
For high-precision work, the most effective method is electrochemical cleaning. This typically involves cycling the electrode's potential in a clean, dilute acid solution (like 0.5M H₂SO₄). This process oxidatively strips away contaminants and then reduces the platinum oxide layer formed during the cleaning, leaving a pristine, highly active surface known as a "platinized" surface.
Understanding the Trade-offs and Best Practices
Careless cleaning can do more harm than good. A platinum electrode is a precision instrument and must be treated as such.
The Dangers of Mechanical Cleaning
Never use abrasives, such as polishing alumina, powders, or rough cloths, for routine cleaning. Platinum is a soft metal. Mechanical abrasion will scratch the surface, altering its roughness factor and effective surface area, which permanently compromises the accuracy of future measurements.
Choosing the Right Chemical Cleaner
Be extremely cautious with chemical agents. While dilute acids are standard, you must avoid any chemical that attacks platinum, most notably aqua regia (a mixture of nitric and hydrochloric acids). Also, avoid cleaners containing ions (like chlorides in some situations) that could interfere with your subsequent experiments.
Inspect Before Every Use
Before an experiment, always visually inspect the electrode. The platinum surface should be smooth and bright. A dull or discolored appearance indicates contamination or oxidation and means that more than a simple water rinse is needed.
Proper Handling and Storage
Handle the electrode gently, touching only the non-active parts. When in use, ensure only the platinum tip or wire contacts the electrolyte. After cleaning and drying, store the electrode in a dedicated, clean container where it is protected from physical damage and airborne contaminants.
Making the Right Choice for Your Goal
Your cleaning protocol should match the demands of your work.
- If your primary focus is routine analysis in clean systems: A prompt and thorough rinse with deionized water followed by gentle drying is sufficient for daily use.
- If your work involves organic compounds or greases: Incorporate a solvent rinse with ethanol or isopropanol into your routine before the final water wash.
- If you require the highest accuracy and reproducibility: Perform a pre-experiment electrochemical cleaning cycle in dilute acid to ensure a fully active and standardized surface.
- If your electrode is visibly fouled or has poor performance: Use a dilute acid soak or electrochemical cleaning to restore the surface before proceeding.
A consistently clean electrode is the foundation of reliable electrochemical data.
Summary Table:
| Cleaning Method | Best For | Key Steps |
|---|---|---|
| Immediate Rinse & Dry | Routine use, preventing salt deposits | Rinse with distilled water, blot dry with filter paper |
| Solvent Wash | Organic residues, grease | Rinse with ethanol/isopropanol, then water |
| Acid Soak | Inorganic deposits, metal oxides | Soak in dilute HCl/H₂SO₄, then rinse thoroughly |
| Electrochemical Cleaning | Highest accuracy, complete restoration | Cycle potential in clean, dilute acid solution |
Ensure your electrochemical measurements are always accurate and reliable. Proper electrode care is fundamental to lab success. KINTEK specializes in high-quality lab equipment and consumables, providing the tools and expertise to support your research. If you have questions about maintaining your specific electrode setup or need reliable lab supplies, contact our experts today for personalized support.
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
People Also Ask
- What precautions should be taken when using a platinum sheet electrode? Ensure Accurate & Reproducible Electrochemical Data
- What are the specifications of the Platinum-Titanium Functional Electrode? Maximize Electrochemical Performance
- What are the available specifications for platinum sheet electrodes? Find the Perfect Fit for Your Electrochemical Needs
- How should a platinum sheet electrode be pretreated before use? Ensure Accurate Electrochemical Measurements
- How should a platinum sheet electrode be operated during an experiment? Ensure Accurate and Reproducible Results



















