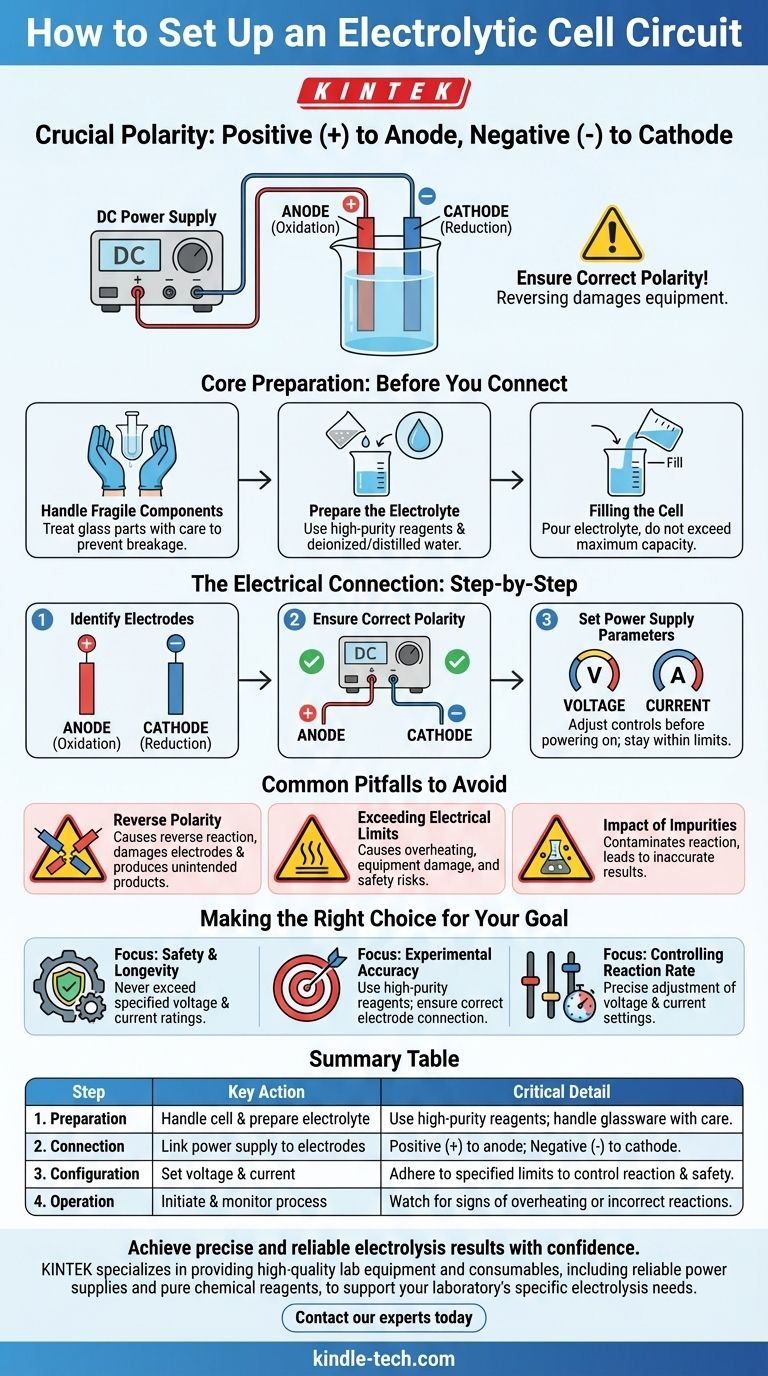
To properly set up the electrical circuit for an electrolytic cell, you must connect the positive terminal of the power supply to the cell's anode and the negative terminal to the cell's cathode. It is absolutely crucial to ensure this polarity is correct before applying power. This connection drives the non-spontaneous chemical reaction central to electrolysis.
A successful and safe electrolytic setup goes beyond just connecting wires. It requires careful preparation of the electrolyte, meticulous handling of fragile components, and strict adherence to the specified voltage and current limits to control the reaction and prevent damage.

Core Preparation: Before You Connect Anything
Before dealing with the electrical circuit, proper physical and chemical preparation is essential for a safe and effective experiment.
Handling Fragile Components
The electrolytic cell is typically made of glass, which is fragile. Always handle the components with care to prevent breakage, holding them gently and securely.
Preparing the Electrolyte
The quality of your electrolyte directly impacts the reaction. Use high-purity chemical reagents and deionized or distilled water to avoid introducing impurities that could cause unwanted side reactions.
Filling the Cell
Pour the prepared electrolyte into the cell carefully. Ensure the final volume does not exceed the maximum capacity line to prevent spills or overflow during operation.
The Electrical Connection: A Step-by-Step Guide
With the cell prepared, you can now focus on the electrical circuit. Precision here is non-negotiable.
Identify Your Electrodes
An electrolytic cell has two electrodes: the anode (where oxidation occurs) and the cathode (where reduction occurs).
Ensure Correct Polarity
The entire process relies on forcing a reaction with external power.
Connect the positive (+) terminal of your DC power supply to the anode.
Connect the negative (-) terminal of your DC power supply to the cathode. Double-check these connections before proceeding.
Set Your Power Supply Parameters
Before turning on the power, adjust the controls. The electrochemical reaction can be precisely managed by setting the correct voltage and current.
Common Pitfalls to Avoid
Mistakes in the setup can lead to failed experiments, damaged equipment, or safety hazards.
The Risk of Reverse Polarity
Connecting the terminals incorrectly will reverse the intended electrochemical reaction. This can damage the electrodes or produce unintended chemical products.
The Danger of Exceeding Electrical Limits
Never exceed the rated current and voltage specified for your particular electrolytic cell and power supply. Doing so can cause overheating, damage the equipment, and create a significant safety risk.
The Impact of Impurities
Failing to use pure reagents for your electrolyte can contaminate the reaction. This leads to inaccurate results and can interfere with the desired chemical process.
Making the Right Choice for Your Goal
Your specific objective will determine which aspect of the setup requires the most attention.
- If your primary focus is safety and equipment longevity: Never exceed the specified voltage and current ratings for your power supply and cell.
- If your primary focus is experimental accuracy: Ensure you use high-purity reagents for the electrolyte and that the anode and cathode are connected to the correct terminals.
- If your primary focus is controlling the reaction rate: Pay close attention to the adjustable voltage and current settings on your power supply, as these directly govern the process.
A methodical and careful approach to setup is the foundation of any successful electrolysis experiment.
Summary Table:
| Step | Key Action | Critical Detail |
|---|---|---|
| 1. Preparation | Handle cell & prepare electrolyte | Use high-purity reagents; handle glassware with care. |
| 2. Connection | Link power supply to electrodes | Positive (+) to anode; Negative (-) to cathode. |
| 3. Configuration | Set voltage & current | Adhere to specified limits to control reaction and ensure safety. |
| 4. Operation | Initiate and monitor the process | Watch for signs of overheating or incorrect reactions. |
Achieve precise and reliable electrolysis results with confidence.
Setting up your electrolytic cell correctly is fundamental to experimental success and safety. KINTEK specializes in providing high-quality lab equipment and consumables, including reliable power supplies and pure chemical reagents, to support your laboratory's specific electrolysis needs.
Contact our experts today to ensure your setup is optimized for accuracy, safety, and performance. Let us help you select the right equipment for your application.
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Electrolytic Electrochemical Cell for Coating Evaluation
- Thin-Layer Spectral Electrolysis Electrochemical Cell
People Also Ask
- What is the structure of an H-type exchangeable membrane electrolytic cell? A Guide to Precise Electrochemical Separation
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments
- How should the H-type electrolytic cell be stored when not in use? Expert Storage & Maintenance Guide
- What optical features does the H-type electrolytic cell have? Precision Quartz Windows for Photoelectrochemistry
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup















