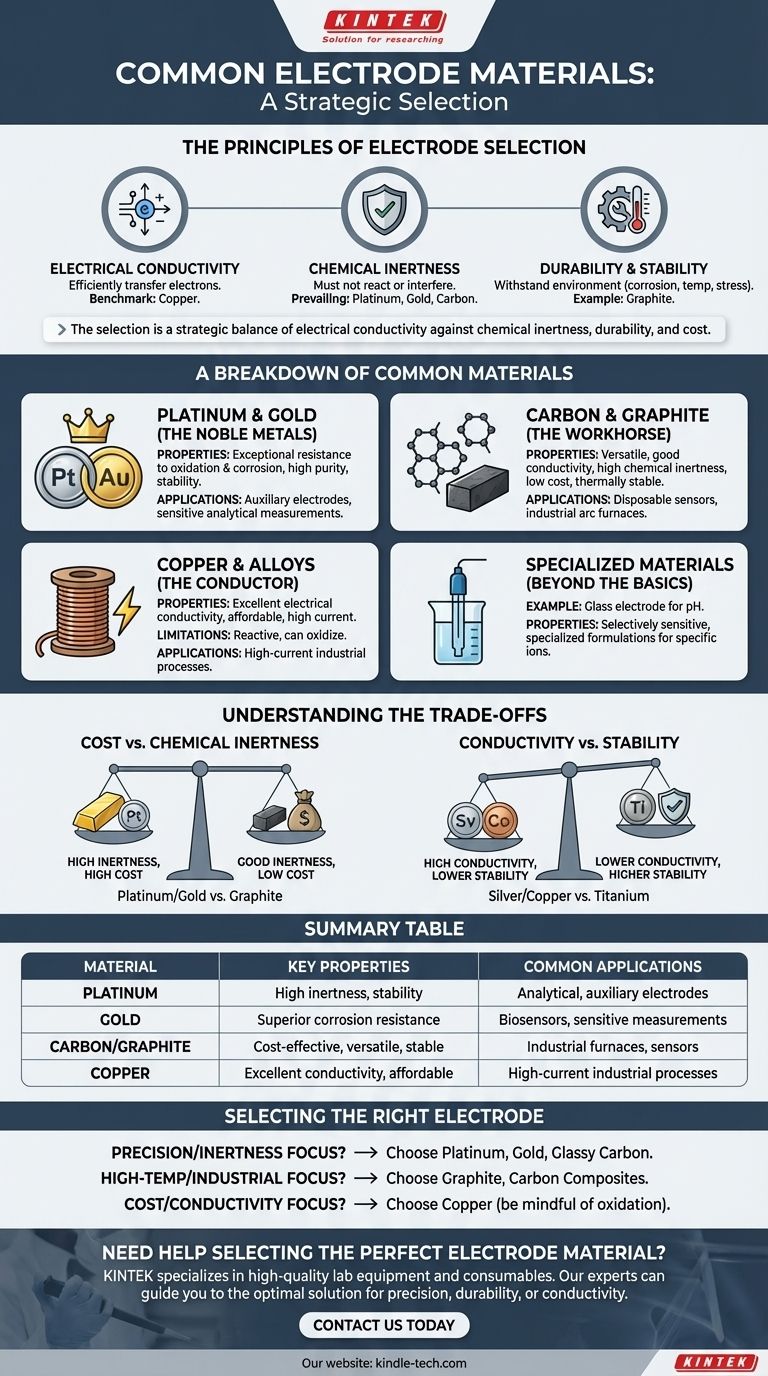
The most common electrode materials are platinum, gold, carbon (in the form of graphite), and copper. These materials are selected for their distinct properties, with platinum and gold valued for chemical inertness, carbon for its versatility and cost-effectiveness, and copper for its excellent electrical conductivity at a moderate price.
The selection of an electrode material is rarely about finding a single "best" option. It is a strategic decision that balances the non-negotiable need for electrical conductivity against the specific demands of the application, primarily chemical inertness, durability, and cost.

The Principles of Electrode Selection
To understand why certain materials are used, we must first examine the core properties that define a functional electrode. The relative importance of these factors changes dramatically depending on the electrochemical process.
Electrical Conductivity
An electrode's primary function is to conduct electricity, making high electrical conductivity a fundamental requirement. The material must efficiently transfer electrons between the external circuit and the chemical species in the solution.
Copper is a benchmark for this property, second only to silver in bulk conductivity. However, pure conductivity is never the only factor.
Chemical Inertness
In many applications, especially in analytical chemistry or as an auxiliary (counter) electrode, the electrode must not react with the electrolyte or interfere with the primary reaction. Its role is simply to complete the electrical circuit.
This is why platinum, gold, and carbon are so prevalent. They are electrochemically inert across a wide range of conditions, ensuring that they do not corrode, dissolve, or catalyze unwanted side reactions.
Durability and Stability
An electrode must withstand its operating environment. This includes resistance to chemical corrosion (oxidation), high temperatures, and mechanical stress.
For example, electrodes in arc melting furnaces are made of graphite or carbon because these materials can endure extreme thermal shock and remain physically stable at temperatures that would melt most metals.
A Breakdown of Common Electrode Materials
Different materials serve different roles based on their unique combination of properties. They can be grouped into logical categories.
The Noble Metals: Platinum and Gold
Platinum and gold are the premium choices for electrodes. Their exceptional resistance to oxidation and corrosion makes them ideal for applications demanding the highest purity and stability.
They are the standard for auxiliary electrodes and many working electrodes in sensitive analytical measurements where any interference from the electrode itself would compromise the results.
The Workhorse: Carbon and Graphite
Carbon, most often in the form of graphite, is arguably the most versatile electrode material. It offers a strong combination of good electrical conductivity, high chemical inertness, and a much lower cost than noble metals.
Its utility ranges from disposable screen-printed electrodes in sensors to massive blocks in industrial arc furnaces. Its availability and machinability add to its practical appeal.
The Conductor: Copper and its Alloys
When the primary requirement is high current efficiency and cost is a major consideration, copper is a dominant choice. Its excellent conductivity makes it ideal for carrying large currents.
However, copper is more reactive than noble metals or carbon and can oxidize (corrode). This makes it unsuitable for many analytical applications but perfectly acceptable for certain industrial processes or as a substrate for other materials.
Specialized Materials: Beyond the Basics
Some applications require highly specialized materials. A common example is the glass electrode used for pH measurements, which is made from a doped glass formula that is selectively sensitive to hydrogen ions.
Understanding the Trade-offs
Choosing an electrode material always involves balancing competing priorities. There is no universally perfect material.
Cost vs. Chemical Inertness
This is the most frequent trade-off. Platinum and gold offer near-perfect inertness but come at a significant cost. Graphite, while slightly less inert under extreme conditions, provides excellent performance for a fraction of the price, making it the practical choice for a huge range of applications.
Conductivity vs. Stability
A material with superior conductivity may lack chemical or physical stability. Silver has the highest electrical conductivity of any metal, but copper is often preferred because it is stronger and more affordable.
However, copper itself is less resistant to oxidation than titanium, which might be chosen in a corrosive environment despite its lower conductivity. The "best" choice depends on which property is the bottleneck for performance.
The Role of the Electrode
The material requirements change based on the electrode's function in the cell. A working electrode, where the reaction of interest occurs, has different needs than an auxiliary electrode, whose only job is to support the current flow without interfering. Any material suitable for a working electrode can be used as an auxiliary, but the reverse is not true.
Selecting the Right Electrode for Your Application
Your choice should be guided by your primary objective.
- If your primary focus is analytical precision or preventing reaction interference: Choose platinum, gold, or glassy carbon for their superior chemical inertness.
- If your primary focus is high-temperature industrial processing: Choose graphite or other carbon-based composites for their unique thermal and mechanical stability.
- If your primary focus is general-purpose use where cost and high conductivity are key: Choose copper, but be mindful of its potential for oxidation in your specific environment.
By understanding these core trade-offs, you can confidently select an electrode material that is not just common, but correct for your specific goal.
Summary Table:
| Material | Key Properties | Common Applications |
|---|---|---|
| Platinum | High chemical inertness, excellent stability | Analytical chemistry, auxiliary electrodes |
| Gold | Superior corrosion resistance, non-reactive | Sensitive analytical measurements, biosensors |
| Carbon/Graphite | Cost-effective, versatile, thermally stable | Industrial furnaces, disposable sensors |
| Copper | Excellent electrical conductivity, affordable | High-current industrial processes, general use |
Need help selecting the perfect electrode material for your lab? At KINTEK, we specialize in providing high-quality lab equipment and consumables, including electrodes tailored to your specific research or industrial needs. Whether you require the precision of platinum, the durability of graphite, or the conductivity of copper, our experts can guide you to the optimal solution. Contact us today to discuss your application and discover how KINTEK can enhance your laboratory's performance and efficiency.
Visual Guide
Related Products
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Iridium Dioxide IrO2 for Water Electrolysis
- Nickel Aluminum Tabs for Soft Pack Lithium Batteries
- Li-Air Battery Case for Battery Lab Applications
People Also Ask
- What is the application of RRDE? Unlock Quantitative Catalyst and Reaction Insights
- What is a common application for the platinum wire/rod electrode? The Essential Guide to Counter Electrodes
- What is the rotating ring disk electrode method? Unlock Real-Time Reaction Analysis
- What is the difference between ring disk electrode and rotating disk electrode? Unlock Deeper Electrochemical Insights
- What is the difference between RDE and RRDE? Unlock Advanced Electrochemical Reaction Analysis



















