In short, the conditions of any heat treatment process are defined by three primary variables: the temperature the metal is heated to, the time it is held at that temperature, and the rate at which it is cooled. These factors are precisely manipulated to alter the metal's internal microstructure, thereby changing its physical and mechanical properties, such as hardness, strength, and ductility.
The core principle of heat treatment is not just about heating and cooling metal. It's about using temperature, time, and cooling rate as precise levers to control phase transformations within the material's crystal structure to achieve a specific, desired engineering outcome.

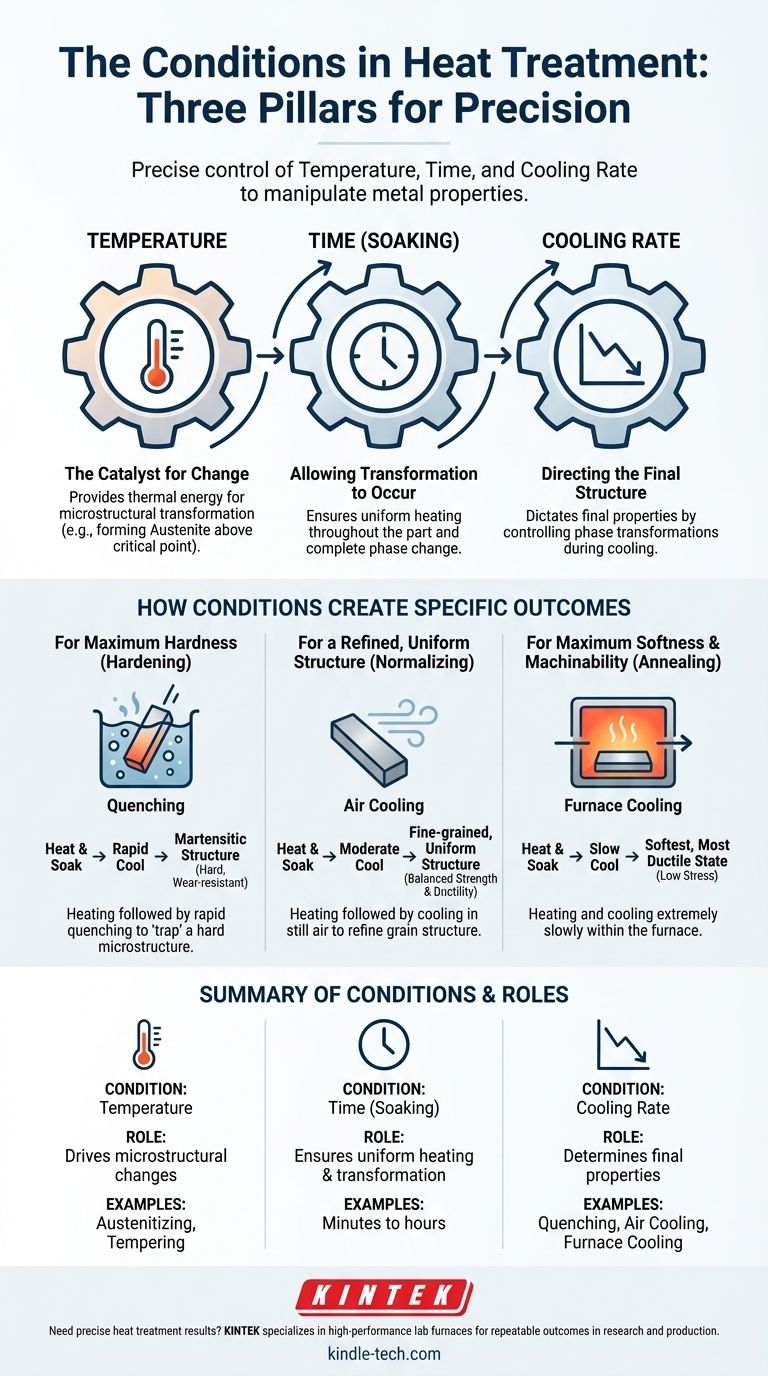
The Three Pillars of Heat Treatment
Every heat treatment cycle, from simple stress relieving to complex hardening, is built upon the careful control of three fundamental conditions. Understanding how each one works is key to understanding the entire process.
Temperature: The Catalyst for Change
Temperature provides the thermal energy required for atoms within the metal's crystal lattice to move and rearrange.
When a steel part is heated past a critical point (its austenitizing temperature), its fundamental crystal structure transforms. This new, high-temperature structure (austenite) is the necessary starting point for most strengthening heat treatments.
Time: Allowing Transformation to Occur
Reaching the target temperature is not enough. The metal must be held at that temperature, a step known as soaking, for a specific duration.
This holding time ensures two things: first, that the entire part, from its surface to its core, reaches a uniform temperature. Second, it allows the necessary microstructural changes, like the formation of austenite, to complete throughout the material.
Atmosphere & Cooling Rate: Directing the Final Structure
The final, and often most critical, condition is the cooling phase. The speed at which the metal is cooled from its high-temperature state dictates its final properties.
- Rapid Cooling (Quenching): Plunging a hot part into water, oil, or a polymer solution "traps" a hard, brittle microstructure (martensite). This is the basis of hardening.
- Moderate Cooling (Air Cooling): Allowing the part to cool in still air, as in normalizing, produces a fine-grained, uniform structure with a good balance of strength and ductility.
- Slow Cooling (Furnace Cooling): Leaving the part in the furnace and allowing it to cool down very slowly, as in annealing, produces the softest, most ductile, and lowest-stress state.
The atmosphere within the furnace is also a controlled condition. Using a protective atmosphere, as in normalizing, prevents oxygen from reacting with the hot metal surface, thus avoiding scaling (oxidation) and the loss of carbon (decarburization).
How Conditions Create Specific Outcomes
By manipulating these three pillars, metallurgists can achieve the specific benefits needed for an application.
For Maximum Hardness (Hardening)
To make steel hard and wear-resistant, you use conditions that create a martensitic structure. This involves heating above the critical temperature, soaking, and then cooling as rapidly as possible without cracking the part.
For Maximum Softness and Machinability (Annealing)
To prepare a part for extensive machining or to relieve stresses from welding or forming, you need the softest possible state. This is achieved by heating, soaking, and then cooling the part extremely slowly, often over many hours inside the furnace.
For a Refined, Uniform Structure (Normalizing)
After a process like hot forging, a metal's grain structure can be inconsistent and large, which can lead to unpredictable properties. Normalizing refines and homogenizes this structure by heating the part and letting it cool in open air, which is faster than annealing but much slower than quenching.
Understanding the Trade-offs
Choosing heat treatment conditions is always a matter of balancing competing properties.
The Hardness vs. Brittleness Dilemma
Achieving maximum hardness through quenching almost always results in high brittleness. A fully hardened part may shatter under impact. This is why a secondary, low-temperature treatment called tempering is almost always performed after hardening to restore some ductility and toughness, albeit at the cost of some hardness.
The Risk of Distortion and Cracking
Rapid changes in temperature create immense internal stresses. During a fast quench, the surface of a part cools and shrinks much faster than its core. This differential can be so severe that it causes the part to warp, distort, or even crack.
The Impact of Part Geometry
The thickness and complexity of a part heavily influence the effectiveness of heat treatment. A thick section will never cool as quickly at its core as it does at its surface, meaning it's impossible to achieve the same level of hardness all the way through with a standard quench.
Matching the Conditions to Your Goal
The right conditions are entirely dependent on the final goal for your component.
- If your primary focus is creating a wear-resistant component: Your conditions must include heating to the proper austenitizing temperature followed by a rapid quench to maximize hardness.
- If your primary focus is preparing a part for easy machining or forming: Your conditions should be those for annealing, defined by a very slow cooling rate to achieve maximum softness.
- If your primary focus is improving the structural uniformity after welding or forging: Your conditions should be for normalizing, which uses a moderate cooling rate in air to refine the grain structure.
By mastering these fundamental conditions, you gain direct control over the final performance and reliability of your metallic components.
Summary Table:
| Key Condition | Role in Heat Treatment | Common Examples |
|---|---|---|
| Temperature | Drives microstructural changes (e.g., austenite formation) | Austenitizing, annealing, tempering |
| Time (Soaking) | Ensures uniform heating and complete transformation | Minutes to hours, depending on part size |
| Cooling Rate | Determines final properties (hardness, ductility) | Quenching (rapid), air cooling (moderate), furnace cooling (slow) |
Need precise heat treatment results for your lab or production? KINTEK specializes in high-performance lab furnaces and equipment that deliver exact control over temperature, time, and atmosphere. Whether you're hardening, annealing, or normalizing, our solutions ensure repeatable outcomes for metals research, quality control, and manufacturing. Contact our experts today to optimize your heat treatment processes!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What are the uses of vacuum furnace? Achieve Unmatched Material Purity and Performance
- Why do you vacuum for heat treatment? Achieve Flawless, High-Performance Metal Components
- Where are vacuum furnaces used? Essential for High-Purity Heat Treatment in Critical Industries
- What is the standard thickness of plating? Optimize Durability, Corrosion & Cost
- What is the leak rate for a vacuum furnace? Ensure Process Purity and Repeatability



















